Case Law Details
In re BASF India Ltd. (CAAR Mumbai)
CAAR held that Luprosil salt is classifiable under heading 2915 and more specifically, under subheading 29155000 of the first schedule to the Customs Tariff Act, 1975.
FULL TEXT OF THE ORDER OF CUSTOMS AUTHORITY OF ADVANCE RULING, MUMBAI
M/s. BASF India Ltd. (the applicant, in short) has filed an application for advance ruling on 16.04.2022 seeking an advance ruling on the classification of Luprosil Salt Calcium. The applicant has stated that the import would be from their German parent and other parties. The product is a formulation in the form of powder with 98% calcium propionate, equivalent to approx. 77% propionic acid and approx. 21% calcium and acts as feed preservative by inhibiting mould growth. It is stated that, propionic acid has a pronounced anti-microbial effect and acts on moulds, yeast and bacteria. The product would be used in animal feed in the form in which it is imported and has no other use, either as medicine or for the manufacture of medicine for humans. The global desk of the applicant has been classifying the product as organic acids under HS code 29.15. However, as per the applicant’s understanding, on account of the fact that the product in question is used for a very specific purpose, i.e., preparation of animal feed, classification under heading 23.09 of the customs tariff as premix for animal feed is also a possibility and the domestic industry is also classifying similar products under the heading 23.09 of the tariff. Thus, considering the difference in views between the applicant’s global desk and the practice followed by the domestic industry, the present application is preferred. The applicant intends to import the said goods through the entry points of seaports/airports at Nhava-Sheva, Mumbai (Air Cargo Complex), Chennai (preventive commissionerate) and Kolkata. Accordingly, comments from the jurisdictional Principal Commissioners/ Commissioners of Customs were invited.
2. The applicant has submitted that the end use would only be in animal feed preparations and are labelled, marketed and considered by the trade as intended for use only in preparation of animal feed. This fact has also been clarified by the Department of Animal Husbandry, Dairying and Fisheries and the clarificatory letter written to the Drug Controller by the Department of Animal Husbandry also states the same.
3. The customs preventive commissionerate of Chennai, in their comments, has stated that there is no clarity about the preparation process of the item Luprosil salt except that it is chemically named as calcium propionate or calcium di-propionate and prepared by using calcium hydroxide and propionic acid. Since, it is basically a salt of propionic acid, as per the Indian customs tariff, it has to be classified under the sub-heading 29155000. As per the HSN explanatory notes, chapter 23.09 includes products of a kind used in animal feeding, not elsewhere specified or included, obtained by processing vegetable or animal materials to such an extent that they have lost the essential characteristics of the original material, other than vegetable waste, vegetable residues and by-products of such processing. But, Luprosil salt is not obtained from any animal or vegetable materials. Moreover, it can be classified under the heading 2309 only when it is not mentioned or specified anywhere else. Calcium Propionate is basically used as food preservative and cannot form a complete feed for the animal. As per the HSN explanatory notes, even though preparations designed to preserve the feeding stuffs can be classified under the heading 2309, it should be a compound composition (additives or premixes) consisting of a number of substances like vitamins, minerals, organic and inorganic matter, antibiotics, anti-oxidants, stabilizers etc and should form a complete animal feed.
3.1 Further as per note 1 (a) to chapter 29, separate chemically defined organic compounds, whether or not containing impurities are classifiable under chapter 29. Thus, Luprosil salt being a separate chemically defined organic compound, is classifiable under the chapter 29 and not under the chapter 23. M per the HSN explanatory notes, the term “premix” under the heading 2309 has to be a mixture of the following three items: –
(1) Those which improve digestion and, more generally, ensure that the animal makes good use of the feeds and safeguard its health: – vitamins or pro-vitamins, amino-acids, antibiotics, coccidiostats, trace elements, emulsifiers, flavourings and appetisers, etc.
(2) Those designed to preserve the feeding stuffs (particularly the fatty components) until consumption by the animal: – stabilisers, anti-oxidants, etc.
(3) Those which serve as carriers and which may consist either of one or more organic nutritive substances (manioc or soya flour or meal, middlings, yeast, various residues of the food industries, etc.) or of inorganic substances (e.g., magnesite, chalk, kaolin, salt, phosphates).
3.2 Therefore, Luprosil salt, used to preserve the feeding stuffs, cannot be accepted as animal feed/premix unless mixed with other additives, organic matters, carriers etc. Reliance has also been placed on the landmark Supreme Court judgement in the case of Dilip Kumar (https://www.cbic.gov.in/resources//htdocs-cbec/legalaffairs/Dilip Kumar&Co.pdf) wherein it has held that even if the items are used in animal feed (vitamin E in that case) they have to be classified as per the provisions of Indian customs tariff, chapter notes, HSN explanatory notes, headings etc.
4. Personal hearing was held on 01.06.2022 in virtual mode along with four other applications on the matter of classification of animal feed/supplements filed by the same applicant. Shri. T. Vishwanathan and others represented the applicants. However, no one was present for the department/jurisdictional commissionerates. Shri. Vishwanathan and his colleagues explained the technical/safety data sheets, manufacturing process, composition and product label of the products. They have also submitted a compilation containing, inter-alia, notifications/circulars, cross rulings, case laws etc. My attention was specifically drawn to the decision of Hon’ble Tribunal in the case of Lalchand Bhimraj & Tetragon Chemie as well as the CBEC circular of 26.03.1996. Shri. Vishwanathan was informed that comments have been received from one jurisdictional commissioner which has been send to them and they should expedite their rejoinder. Shri. Vishwanathan promised to expedite the same and stated that they would be submitting another compilation at the earliest.
5. The applicant additionally submitted an excel sheet containing the end use of the product and its inactive ingredients.
Table 1
|
Product name |
Whether the product contains additives (Yes/No) | Active Ingredients | Inactive ingredie nts | Usage of inactive ingredients | End-use of the product |
| Luprosil Salt | No | 98% Calcium Propionate equivalent to Approx. 77% Propionic Acid, Approx. 21% Calcium | N/A | N/A | It is used in the compound feed & raw material preservation. It can be added in silage for long term storage. It reduces microbial content in feed stuff, prevent spoilage, fungal growth, heating up, lumping & nutrient losses. |
In the said additional submissions/rejoinder, the applicant has submitted that as per their understanding, the impugned product is correctly classifiable under heading 23.09 of the customs tariff as “Preparations of a kind used in animal feeding”. They have quoted the HSN Explanatory Notes and have placed reliance on following case laws:
Reckitt and Colman of India Ltd. vs. CCE – 1985 (22) ELT 216 (Tribunal) has held that to qualify as a preparation the said product should be prepared by addition, mixing or such other similar process to the original commodity in order to derive a new commodity.
Kothari Chemicals vs. Union of India – 1996 (86) E.L.T. 209 (All.), wherein the Hon’ble Allahabad High Court described preparations as “products which are made from separate components”.
The applicant has also submitted the manufacturing flow chart as below:
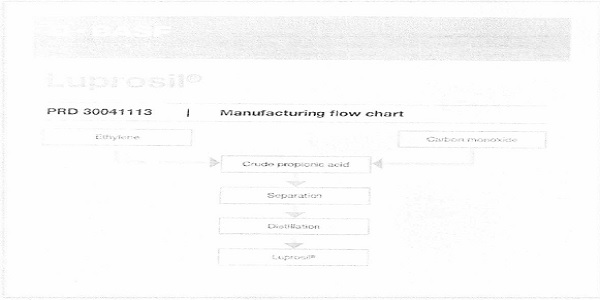
6. I have considered all the materials placed before me in respect of the subject product. I have gone through the submissions made by the applicant during the personal hearing. I proceed to pronounce my ruling on the basis of information available on record as well as information gathered from other reliable sources. The product, Luprosil salt is either calcium propionate or calcium di-propionate. The composition of Luprosil salt is almost 98 % of calcium propionate, which is equivalent to approx. 77% propionic acid and approx. 21% Calcium. As per the applicant, the product is used for a very specific purpose, i.e., preparation of animal feed, which merit classification under heading 2309. However, Luprosil salt, a salt of propionic acid, finds specific entry under sub- heading 2915 50 00 —”Propionic acid, its salts and esters”. Therefore, heading 2915, which is adopted by the global desk of the applicant, is the competing classification entry. The relevant entries for classifications are reproduced below:
2309 Preparations of a kind used in animal feeding
2915 Carboxylic acids and their anhydrides, halides, peroxides and peroxyacids and their halogenated, sulphonated, nitrated or nitrosatedderivatives; saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives
29155000 Propionic acid, its salts and esters
6.1 Note to Chapter 23 states that “Heading 2309 includes products of a kind used in animal feeding, not elsewhere specified or included, obtained by processing vegetable or animal materials to such an extent that they have lost the essential characteristics of the original material, other than vegetable waste, vegetable residues and by-products of such processing”. As per HSN explanatory notes to the heading 2309 the heading covers three types of animal feedstuff. First, complete feed, contains energy nutrients, bodybuilding protein nutrients or minerals and function nutrients. The second type of animal feedstuff, i.e., secondary feed, is the preparations devised to compensate for deficiencies, so as to ensure a well-balanced animal diet, consisting of proteins, minerals or vitamins plus additional-energy feeds. The preparations of the third category, also known as premixes, are used in making the complete feed or supplementary feed. From the chapter note and HSN explanatory notes, it is clear that for a product to be a complete feed or a supplementary feed, it needs to have composition of energy nutrients, body-building protein-rich nutrients and function nutrients. From the composition of the said product, as mentioned in Table 1, it is evident that it lacks above mentioned composition. Therefore, Luprosil salt is neither a complete feed or a supplementary feed.
6.2 In respect of third type of feedstuff, premixes, the HSN explanatory note H (C) to heading 2309 elaborates as below:
These preparations, known in the trade as “premixes”, are, generally speaking, compound compositions consisting of a number of substances (sometimes called additives) the nature and proportions of which vary according to the animal production required. These substances are of three types:
(1) Those which improve digestion and, more generally, ensure that the animal makes good use of the feeds and safeguard its health: vitamins or provitamins, amino acids, antibiotics, coccidiostats, trace elements, emulsifiers, flavourings and appetisers, etc.
(2) Those designed to preserve the feeding stuff (particularly the fatty components) until consumption by the animal: stabilisers, anti-oxidants, etc.
(3) Those which serve as carriers and which may consist either of one or more organic nutritive substances (manioc or soy flour or meal, middlings, yeast, various residues of the food industries, etc.) or of inorganic substances (e.g., magnesite, chalk, kaolin, salt, phosphates).
The concentration of the substances described in (1) above and the nature of the carrier are determined so as to ensure, in particular, homogeneous dispersion and mixing of these substances in the compound feeds to which the preparations are added.
Therefore, premixes are compound compositions consisting of a number of substances, which include nutrient, preservative and carrier substances. In the instant case, the product only contains preservative. It neither has nutrient additive or carrier additive. Further, the percentage of active ingredient, i.e., propionic acid/ calcium propionate is very high (98%-100%). Propionic acid and calcium propionate, has applications in, both, food and feed industry as preservatives. From the composition of the product, it does not appear to have specific use as feed additive only. Therefore, it appears that the said product is not a premix for animal feedstuff. Thus, the product does not merit classification under heading 2309.
6.3. Propionic acid or calcium propionate find specific entry under heading 2915. Chapter 29 covers organic chemicals. Note 1 to said chapter states as below:
1. Except where the context otherwise requires, the headings of this Chapter apply only to.
(a) Separate chemically defined organic compounds, whether or not containing impurities;
(b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (Chapter 27);
(c) The products of headings 29.36 to 29.39 or the sugar ethers, sugar acetals and sugar esters, and their salts, of heading 29.40, or the products of heading 29.41, whether or not chemically defined;
(d) The products mentioned in (a), (b) or (c) above dissolved in water;
(e) The products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use;
(f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabiliser (including an anti-caking agent) necessary for their preservation or transport;
(g) The products mentioned in (a), (b), (c), (d), (e) or (0 above with an added anti-dusting agent or a colouring or odoriferous substance or an emetic added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use;
In the present case, as per the applicant, the product contains 98% of Calcium Propionate, a separately defined chemical compound. As per the manufacturing flowchart, it contains distilled propionic acid, a separately defined chemical compound. No other ingredient has been added to the said product.
6.4 HSN explanatory notes to Chapter 29 state that “Separate chemically defined compounds containing other substances deliberately added during or after their manufacture (including purification) are excluded from this Chapter…The separate chemically defined compounds of this Chapter may contain impurities (Note 1 (a))…The term “impurities” applies exclusively to substances whose presence in the single chemical compound results solely and directly from the manufacturing process (including purification). These substances may result from any of the factors involved in the process and are principally the following: (a) Unconverted starting materials, (b) Impurities present in the starting materials, (C) Reagents used in the manufacturing process (including purification). (d) Byproducts. It should be noted, however, that such substances are not in all cases regarded as “impurities” permitted under Note 1 (a). When such substances are deliberately left in the product with a view to rendering it particularly suitable for specific use rather than for general use, they are not regarded as permissible impurities. For example, a product consisting of methyl acetate with methanol deliberately left in with a view to improving its suitability as a solvent is excluded (heading 38.14). For certain compounds (e.g., ethane, benzene, phenol, pyridine), there are specific purity criteria, indicated in Explanatory Notes to headings 29.01, 29.02, 29.07 and 29.33.”
In the present case, the starting material, as provided in the product brochure, is propionic acid. Luprosil salt based on propionic acid does not appear to have any impurity. The 2% unexplained component in Luprosil salt could have resulted from the manufacturing process, having no stated specific utility. Therefore, the product can be classifiable under Chapter 29 and specifically under heading 2915.
6.5 I further find that heading 2309 is in the nature of a residuary heading as note Ito chapter 23 says that the heading 2309 includes products of a kind not elsewhere specified or included, whereas sub-heading 29155000 is a specific heading for propionic acid, its salts and esters. As per the Rule 3 (a) of General Interpretation Rules, a specific heading shall prevail over the general heading. In this regard, the Hon’ble Supreme Court in the case of DUNLOP INDIA LTD & MADRAS RUBBER FACTORY LTD Vs UNION OF INDIA AND OTHERS reported in 2002-TIOL-647-SC-CUS-LB, has held as follows:
“When an article has, by all standards, a reasonable claim to be classified under an enumerated item in the Tariff Schedule, it will be against the very principle of classification to deny it the parentage and consign it to an orphanage of the residuary clause”
6.6. The applicant has placed reliance on the order of the larger bench of the Hon’ble Tribunal in the case of Tetragon Chemie reported at 2001 (138) E.L.T. 414 (Di. -LB) which was upheld by the Hon’ble Supreme Court as reported at 2001 (132) E.L.T. 525(S.C). However, the products in the present case (organic acid of high concentration) is different from that of Tetragon Chemie (premixes of vitamins along with other constituents which was already in the final form of animal feed supplement). The Hon’ble Supreme Court, in the case of CCE, Calcutta vs. Alnoori Tobacco Products reported at 2004-TIOL-85-SC-CX, held that:
“13. Circumstantial flexibility, one additional or different fact may make a world of difference between conclusions in two cases. Disposal of cases by blindly placing reliance on a decision is not proper.
14. The following words of Lord Denning in the matter of applying precedents have become locus classicus:
“Each case depends on its own facts and a close similarity between one case and another is not enough because even a single significant detail may alter the entire aspect, in deciding such cases, one should avoid the temptation to decide cases (as said by Cordozo) by matching the colour of one case against the colour of another. To decide therefore, on which side of the line a case falls, the broad resemblance to another case is not at all decisive. “
“Precedent should be followed only so far as it marks the path off ustice, but you must cut the dead wood and trim off the side branches else you will find yourself lost in thickets and branches. My plea is to keep the path to justice clear of obstructions which could impede it. “”
6.7. Further, the Hon’ble High Court of Allahabad in the case of Sonam International, reported at 2012 (275) E.L.T. 326 (All.), held that vitamins of high concentration used for manufacture of animal feed will be classified under 2936. Admittedly, the product in this case has a very high concentration to the tune of 98%.
6.8. Even though, the applicant in their application has stated that the product which they propose to import is going to be used in the manufacture of animal feed, as discussed in the preceding paragraphs, propionic acid and its salts are not excluded from the ambit of the chapter 29. On the contrary, as already discussed above, there is a very specific tariff entry for this very product. Therefore, when confronted with a specific classification entry vis-à-vis a residuary classification entry, one must favour the specific entry. In fact, as per the mandate of rule 3A of the General Rules of Interpretation of Customs Tariff, the specific heading prevails over the general heading.
7. In view of the foregoing discussions, I rule that the Luprosil salt is classifiable under heading 2915 and more specifically, under subheading 29155000 of the first schedule to the Customs Tariff Act, 1975.




