Introduction: In a significant move, the Food Safety and Standards Authority of India (FSSAI) has issued an order dated 8th November 2023, focusing on enhancing the quality control mechanism for Fortified Rice, Fortified Rice Kernels (FRK), and premix for FRK. The order comes in response to concerns raised by stakeholders, including the Department of Food and Public Distribution, FRK manufacturers, and Rice Millers, emphasizing the national importance of the Public Distribution System.
Detailed Analysis:
1. Licensing Requirements (Section a): FBOs (Food Business Operators) involved in manufacturing FRK and premix for FRK are now required to obtain only an FSSAI License (Central/State). No new registrations will be allowed. Existing FBOs with FSSAI Registration for these products must apply for Central/State License within three months to continue operations. Failure to do so will result in the removal of FRK and premix for FRK from existing Registration Certificates.
2. Mandatory Testing and Declaration (Section b): Every batch of FRK premix and FRK must undergo testing for relevant parameters as prescribed under the Food Safety and Standards Regulations (FSSR). FBOs must upload test reports on the FoSCoS portal, accompanied by a self-declaration covering details such as the source of iron used and the blending ratio of FRK with rice. Testing must be conducted only through FSSAI-notified laboratories.
3. Traceability Measures (Section c): To ensure traceability, manufacturers of Fortified Rice and FRK are mandated to procure raw materials (FRK and premix) only from FSSAI-licensed vendors. Vendors must provide lab reports of testing, conducted exclusively through FSSAI-notified labs.
4. Immediate Implementation (Section 2): The directions outlined in the order are effective immediately, underlining their urgency and importance. The order concludes with the approval of the Competent Authority.
Conclusion: In conclusion, the FSSAI’s order signifies a proactive approach to strengthen the quality control mechanism for Fortified Rice and its derivatives. The emphasis on licensing, mandatory testing, and traceability measures reflects a commitment to ensuring the safety and quality of food products distributed through the Public Distribution System. Stakeholders, including Commissioners of Food Safety, Food Business Operators, and other associations, need to promptly adhere to the new requirements to ensure compliance with FSSAI regulations.
This detailed analysis provides insights into the key aspects of the FSSAI’s order dated 8th November 2023, highlighting the regulatory changes and their implications for the manufacturing and distribution of Fortified Rice and Fortified Rice Kernels. Industry stakeholders must be proactive in implementing these measures to align with the regulatory framework and contribute to the enhancement of food safety standards in the country.
File No: IEC-34011/1/2021-IEC-FSSAI-Part(6)
Food Safety and Standards Authority of India
(A Statutory Authority established under Food Safety and Standards Act, 2006)
(Regulatory Compliance Division)
FDA Bhawan, Kotla Road, New Delhi— 110002
Dated, the 8th November, 2023
Order
Subject: Strengthening the Quality Control Mechanism of Fortified Rice, Fortified Rice Kernels [FRK] and Premix for FRK – reg.
FSSAI has received references from various stakeholders including Department of Food and Public Distribution, FRK manufacturers and Rice Millers etc. regarding strengthening of quality control mechanism for manufacturing and distribution of FRK and resultant Fortified Rice in the country. The existing regulatory mechanism of manufacturing of FRK and Fortified Rice has been reviewed and the following has been decided in the larger public interest and considering the fact that distribution of Fortified Rice through Public Distribution System is a program of National Importance:
a) FBOs manufacturing FRK and premix for FRK shall be required to obtain only FSSAI License [Central/State] and no new registration shall be allowed.
Further, existing FBOs who are operating under FSSAI Registration for the said products are directed to apply for Central/State License as per the eligibility. A transition time of three months is hereby given to existing Registered FBOs to shift to license and utilize the packaging and labelling materials, if any, failing which, the said two products shall be removed from their existing Registration Certificates [RC] to debar them from further manufacturing under RC, without any further notice.
b) Every batch of FRK premix and FRK manufactured by the concerned Manufacturing FBOs including those manufacturing on Registration Certificates, shall be tested for relevant parameters as prescribed under FSSR and the test reports shall be uploaded on FoSCoS portal by the FBO mandatorily. Further, during uploading of such reports, manufacturers of FRK and premix for FRK shall also submit self-declaration covering the details such as Source of Iron used and the blending ratio of FRK with Rice (i.e 1:50 or 1:100) for every batch of production of FRK on FoSCoS portal [https://foscos.fssai.gov.in]. The testing of FRK premix and FRK shall be carried out only through the FSSAI notified laboratories. [The details of notified labs may be accessed at https://www.fssai.gov.in/cms/food-laboratories.php ] – A user manual to upload lab test report and declaration for source of iron used on FoSCoS portal is annexed.
c) Furthermore to ensure the traceability, Manufacturers of Fortified Rice and FRK shall procure raw materials [FRK and Premix] only from FSSAI licensed vendors with the copy of lab report of the testing of FRK and Premix, tested only through FSSAI notified labs.
2. The above directions shall come into force with immediate effect. This issues with the approval of the Competent Authority.
Enclosure: User Manual to upload lab test reports.
(Inoshi Sharma)
Executive Director (CS)
To
1. Commissioners of Food Safety of all States/UTs and Directors of all Regional Offices, FSSAI
2. All Food Business Operators involved in Manufacturing of FRK and Premix for FRK, Associations and Other Stakeholders
3. CTO, FSSAI- with a request for uploading on the FSSAI website.
Copy for information to:
1. Secretary, DFPD
2. PPS to Chairperson, FSSAI
3. PS to CEO, FSSAI
4. Advisor (Quality Assurance), FSSAI
User Manual for uploading Lab Test Report
Step1: Login to the FoSCoS account and navigate to the “Upload Six Monthly Lab Testing Report” option in the left menu panel, then click “Proceed.”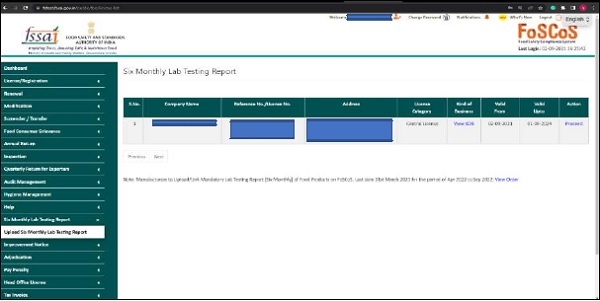
Step 2: Then click on the green button labelled “Upload Six Monthly Lab Testing Report”
Step 3: Select the Financial Year and Session as demonstrated below, and then scroll down.
Step 4: Once you’ve scrolled down, you will find the option of “View & Upload Report” against the FRK and Premix of FRK. Click on “View & Upload Report“
Step 5: Enter the necessary information for your report as shown below.
Step 6: After clicking “Add Report,” you will notice the added report displayed in a row, as illustrated below. You have the option to delete it before making the final submission. Finally, click on “Submit.”
Step 7: Once you’ve submitted the reports, you can access the submitted reports in the “Upload Six Monthly Lab Testing Report” section on the left menu panel. You can repeat the same procedure to add multiple reports for the same product.




