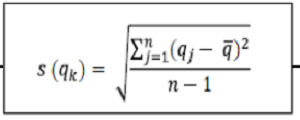Government of India
Ministry of Consumer Affairs, Food & Public Distribution
Department of Consumer Affairs
Legal Metrology Division
Krishi Bhawan, New Delhi-01
I-9/9/2024-W&M Dated: 27.6.2024
Subject- Draft Rules on Breath Analyzer for comments from stakeholders – reg.
Undersigned is directed to refer to the above mentioned subject and to state that the Draft Rules on Breath Analyzer, are placed in the website of the Department www.consumeraffairs.nic.in for seeking comments from all stakeholders by 26.7.2024. The comments may be sent to email-ID: dirwm-ca@nic.in/ ashutosh.agarwal13@nic.in/ mk.naik72@gov.in.
(Ashutosh Agarwal)
Director (Legal Metrology)
Ph: 011-23389489
Email: ashutosh.agarwal13@nic.in
To:
All concerned
[TO BE PUBLISHED IN THE GAZETTE OF INDIA, EXTRA ORDINARY, PART II SECTION 3 SUB-SECTION (i)]
GOVERNMENT OF INDIA
MINISTRY OF CONSUMER AFFAIRS, FOODAND PUBLIC DISTRIBUTION
(DEPARTMENT OF CONSUMER AFFAIRS)
New Delhi, the …………….. 2024
NOTIFICATION
GSR…….. In exercise of the powers conferred by sub-section (1) read with clauses (c), (f), (h), (i) and (s) of sub-section (1) of section 52 of the Legal Metrology Act 2009, (1 of 2010), the Central Government hereby makes the following rules, namely:-
1. Short title and commencement
(1) These rules may be called the Legal Metrology (General) (Amendment) Rules, 2024.
(2) They shall come into force from the date of publication of this notification in the Official Gazette, as follows:
(i) All the Evidential Breath Analyser shall be verified and stamped before sale/ putting into use;
(ii) All the Evidential Breath Analyser which are already in use at the time of publication of this notification shall be verified and stamped within one year.
2. In the index of the Schedule of the Legal Metrology (General) Rules, 2011 under the Eight Schedule after Part XII, the entries “Part XIII”, “Evidential Breath Analyser” shall be inserted.
3. In the Legal Metrology (General) Rules, 2011, (herein after referred to as the said rules), under the Eight Schedule the Part XIII and the entries made thereunder, the following shall be inserted, namely. –
EIGHTH SCHEDULE
SPECIFICATIONS FOR MEASURING INSTRUMENTS
PART XIII- EVIDENTIAL BREATH ANALYSER
Part-1
1. Scope
(1) This Specification applies to evidential breath analyses (EBA), which are quantitative instruments that render a measurement result of alcohol concentration in exhaled human breath for the purpose of establishing compliance, for fighting against alcohol abuse and/or for the advancement of public safety.
(2) These types of instruments are referred to as “evidential” and serve to provide the principal means by which a definitive breath alcohol measurement is obtained.
(3) These devices are not to be confused with those that provide a preliminary result, or that do not quantitatively indicate a measurement result (i.e. pass/fail devices), or that do not provide a sufficiently accurate result to definitively establish a breath alcohol concentration (often referred to as breath alcohol “screening” devices).
(4) For the purpose of this Specification, the term “alcohol” will be used to refer to ethyl alcohol or ethanol in a broader context. However, when dealing with test gas compositions, the exact chemical terminology for each substance will be applied.
(5) Additionally, EBAs may be equipped with special features, for example:
- Prohibiting the displaying or reporting of results that do not represent the final measurement result;
- Mandating the inclusion of a printing device;
- Prohibiting operation of the analyser in the event that no paper is detected in the printing device;
- Requiring further printed information in addition to the final measurement result;
- Requiring final measurement results to be displayed and reported in terms other than the alcohol content in exhaled human breath (i.e. physiological conditions such as ‰ of blood or in terms of other quantities).
(6) The purpose of this Specification is to enumerate the minimum metrological specifications and tests applicable to model approval of quantitative EBAs. It also gives guidance for establishing metrological specifications for initial and re verifications.
(7) The scope of this Specification is limited to the types of EBAs that use mouthpieces for sampling the breath.
2. Terms and definitions
(1) General metrology and legal metrology terms
(i) measurement error:
Measured quantity value minus a reference quantity value
(ii) Adjustment of a measuring system:
Set of operations carried out on a measuring system so that it provides prescribed indications corresponding to given values of a quantity to be measured
(iii) Calibration
Operation that, under specified conditions, in a first step, establishes a relation between the quantity values with measurement uncertainties provided by measurement standards and corresponding indications with associated measurement uncertainties and, in a second step, uses this information to establish a relation for obtaining a measurement result from an indication
(iv) Verification of a measuring instrument
Conformity assessment procedure which results in the affixing of a verification mark and/or issuing of a verification certificate
(v) Verification
Verification of a measuring instrument which has not been verified previously
(vi) Re verification
Verification of a measuring instrument after a previous verification
(vii) Mandatory periodic verification
Re verification of a measuring instrument, carried out periodically at specified intervals according to the procedure laid down
(viii) Putting into service (use)
Moment of the first use by the end-user of a measuring instrument for the purposes for which it was designed
(ix) Being in service (use)
operational life cycle of a measuring instrument after its putting into service, i.e. a measuring instrument in use, after repair, relocated, or rebuilt that may be resold
(x) Disturbance
Influence quantity having a value within the limits specified in this Specification, but outside the specified rated operating conditions of the measuring instrument
Note: An influence quantity is a disturbance if the rated operating conditions for that influence quantity are not specified.
(xi) Fault
Difference between the error of indication and the intrinsic error of a measuring instrument
(xi) Fault limit
Value specified in this Specification delimiting non-significant faults
(xii) Significant fault
Fault exceeding the applicable fault limit
(xiii) Significant defect
Event that has an impact on the properties or functions of the measuring instrument or a fault
(xiv) Intrinsic error
Error of a measuring instrument, determined under reference conditions
(xv) Initial intrinsic error
Intrinsic error of a measuring instrument as determined prior to performance tests and durability evaluations
(xvi) Experimental standard deviation
For a series of n measurements of the same measurand, the quantity s (qk) characterising the dispersion of the results and given by the formula:
With qk being the result of the kth measurement and being the arithmetic mean of the n results considered.
(xviii) Measurement precision
Closeness of agreement between indications or measured quantity values obtained by replicate measurements on the same or similar objects under specified conditions
(xix) Measurement repeatability
Measurement precision under a set of repeatability conditions of measurement
(xx) Repeatability condition of measurement
Condition of measurement, out of a set of conditions that includes the same measurement procedure, same operators, same measuring system, same operating conditions and same location and replicate measurements on the same or similar objects over a short period of time
(xxi) Measurement reproducibility
Measurement precision under reproducibility conditions of measurement
(xxii) Reproducibility condition of measurement
Condition of measurement, out of a set of conditions, that includes different locations, operators, measuring systems, and replicate measurements on the same or similar objects
(xxiii) Stability of a measuring instrument
Property of a measuring instrument, whereby its metrological properties remain constant in time
(xxiv) Uncertainty of a measurement
Non-negative parameter characterizing the dispersion of the quantity values being attributed to a measurand, based on the information used
(xxv) Sensitivity
Quotient of the change in an indication of a measuring system and the corresponding change in a value of a quantity being measured
Note 1: Sensitivity of a measuring system can depend on the value of the quantity being measured.
Note 2: The change considered in a value of a quantity being measured must be large compared with the resolution.
Note 3: In the scope of this Specification, sensitivity relates to the added substance which is not identical with the measurand.
(2) Specific Terms
(i) Evidential breath alcohol analyser (EBA)
Instrument that measures and displays the breath alcohol mass concentration of exhaled human breath within specified error limits
(ii) Stationary evidential breath alcohol analyser (stationary EBA)
Evidential breath alcohol analyser intended only for use in a fixed location within buildings or places providing stable environmental operating conditions
Note: In the scope of this Specification, stationary EBAs are designated as use-case 1.
(iii) Transportable evidential breath alcohol analyser (transportable EBA)
Easily transportable evidential breath alcohol analyser intended for use in mobile applications (e.g. in vehicles)
Note: In the scope of this Specification, transportable EBAs are designated as use-case
(iv) Portable evidential breath alcohol analyser (portable EBA)
Evidential breath alcohol analyser intended for use in outdoor conditions (e.g. handheld devices generally powered by a battery)
Note: In the scope of this Specification, portable EBAs are designated as use-case 3.
(v) Alveolar air
Air contained in the pulmonary alveoli where the gaseous exchange takes place between the blood and the gas contained within the alveoli
(vi) End expiratory breath
Air considered sufficiently representative of alveolar air (as opposed to anatomical dead space)
(vii) Anatomical dead space
Dead space in that portion of the respiratory system which is external to the alveoli and includes the air- conveying ducts from the mouth to the terminal bronchioles
(viii) Measuring mode
Clearly indicated mode in which the EBA can make measurements at the rate normally expected in service and in which it shall meet the performance requirements of this Specification
(ix) Metrological test mode
Mode in which the EBA is subject to metrological control such as verification or adjustment Note: In this mode, more information will be available compared to the measuring mode (e.g. higher resolution, intermediate results, etc.), and access to maintenance and adjustment means is possible.
(x) Standby mode
mode of the EBA whereby only certain circuits are energised in order to conserve power and/or prolong component life, and to attain the measuring mode more rapidly than would be possible if starting from the switched-off state
(xi) Checking facility
Facility that is incorporated in a measuring instrument and which enables significant defects to be detected and acted upon
(xii) Standard measurement cycle
The measurement cycle of an EBA consists of all steps necessary to obtain a valid result, from starting the measurement, sampling, analysing, internal control procedures, calculation, and displaying the result
(xiii) Drift
Continuous or incremental change over time in indication, due to changes in metrological properties of a measuring instrument
(xiv) Memory effect
Effect on the true alcohol concentration of the sample caused by previous samples
(xv) Plateau of alcohol concentration
Time period during exhalation when the alcohol content is considered to reach a nearly stable value
(3) Software Terms
(i) Authenticity
Result of the process of authentication (passed or failed)
(ii) Authentication
Checking of the declared or alleged identity of a user, process, or measuring instrument Note: This may be necessary when checking that downloaded software originates from the owner of the certificate.
(iii) Cryptographic means
Means such as encryption/ decryption with the purpose of hiding information from unauthorised persons, or hashes and signatures to ensure integrity and authenticity
(iv) Error log
Continuous data file containing an information record of failures or significant defects that have an influence on the metrological characteristics of the measuring instrument
(v) Hash function
(Mathematical) function which maps values from a large (possibly very large) domain into a smaller range
(vi) Integrity (of programs, data, or parameters)
Assurance that the programs, data, or parameters have not been subjected to any unauthorised or unintended changes while in use, transfer, storage, repair, or maintenance
(vii) Interface
Shared boundary between two functional units, defined by various characteristics pertaining to the functions, physical interconnections, signal exchanges, and other characteristics of the units, as appropriate
(viii) legally relevant
Attribute of a part of a measuring instrument, a device or software subject to legal control
(ix) Sealing
Means intended to protect the measuring instrument against any unauthorised modification, readjustment, removal of parts, software, etc.
(x) Software examination
Technical operation that consists of determining one or more characteristics of the software according to the specific procedure (e.g. analysis of technical documentation or running the program under controlled conditions)
(xi) Software identification
Sequence of readable characters (e.g. version number, checksum) that represents the software or software module under consideration
(xii) Transmission of measurement data
Electronic transportation of measurement data via communication lines or other means to a receiver where they are further processed
(xiii) User interface
Interface that enables information to be interchanged between the operator and the measuring instrument or its hardware components or software modules
Note: Examples are switches, keyboard, mouse, display, monitor, printer, touch-screen, software window on a screen including the software that generates it.
(4) Abbreviations and symbols
AC : alternating current
AM : amplitude modulation
ASD : acceleration spectral density
DC : direct current
EBA : evidential breath alcohol analyser
EM : electromagnetic
e.m.f. : electromotive force
ESD : electrostatic discharge
EUT : equipment under test
fnom : nominal supply frequency
IEC : International Electrotechnical Committee
MPE : maximum permissible error
RF : radio frequency
RH :L relative humidity
RMS : root mean square
Tamb-low : low ambient temperature
Tamb-high : high ambient temperature
TR : reference temperature
Unom : nominal supply voltage
Ubmin : minimum battery supply voltage
UDC : nominal DC voltage
ethanol mass concentration in the gaseous phase
ethanol mass concentration in the liquid phase
The abbreviations for software validation procedures are explained in Para 6(3)(ii) of Part II of this specification.
3. Description of the Instrument
(1) Schematic description
Generally, an EBA provides a means for sampling and then measuring the alcohol content of a sample of end expiratory breath of a human being. The means for conveying the breath sample through the sampling system depends on the kind of alcohol sensor used in the specific EBA. Incorporated into the sampling system is an alcohol sensor, which analyses the breath sample and provides signals related to the concentration of alcohol. The sensor signals are then electrically processed to display the results of a measurement in mg/L or another prescribed SI unit. Additionally, the EBA has a means to check whether the conditions for the acceptance of a breath sample are fulfilled.
Typically, the major components of an EBA are as follows:
- replaceable mouthpieces to hygienically conduct the breath sample into the EBA for analysis;
- a hose to convey the breath sample through the sampling system or a sampling probe to convey a sub-sample of the breath sample through the sampling system;
- a means to monitor the flowrate, time and volume;
- at least one sensor to measure the alcohol content of the breath sample;
- a data system to process the measurement signal, including an indicating device to display the results and messages;
- an interface to an external data connection;
- a control facility to initiate and check instrument operations; and
- an adjustment facility to set the instrument operating parameters within prescribed limits.
(2) Sampling and mouthpiece
A specimen of an end expiratory breath sample from a continuous and uninterrupted expiration shall be analysed for alcohol concentration. The breath sample shall not be influenced by breathing techniques.
The EBA shall be capable of being used under satisfactory hygienic conditions. This means the use of individually packaged, replaceable mouthpieces for each measurement shall be indispensable.
The mouthpiece shall comply with the requirements of Para2(1)(ix) of Part II
(3) Analysis
The EBA determines the alcohol concentration of the breath sample of end expiratory breath. Influences during the analysis caused by sampling and/or ambient conditions shall be avoided.
(4) Presentation and storage of the result
On a typical EBA, the measurement results will be presented on a display and secured for later access. This could be achieved by either printing or storing the result in the instrument memory, depending on the model as well as the requirements.
(5) Measurement cycle
In general, a measurement cycle of an EBA consists of the following steps:
- Preparation for the measurement/getting ready for sampling;
- Sampling;
- analysis of the sample including internal checking operations; and
- presentation and storage of the result
a complete measurement cycle may consist of one or more breath samples.
4. Units of measurement and decimal sign
The EBA shall display and/or print measurement results in terms of mass concentration of alcohol in a specified volume of exhaled air.
At least in the metrological test mode, the EBA shall be able to indicate the mass concentration in milligram per litre of exhaled breath (mg/L).
The use of an equivalent unit of measurement is possible if the indication is in conformity with SI units.
The decimal marker on the display or printout shall be either a comma or a dot on the line.
Part II
1. Metrological Requirements
(1) Measuring range
The measuring range of the EBA shall be from 0.00 mg/L to at least 2.00 mg/L.
A greater upper limit of the measuring range may be defined by the manufacturer. The EBA shall indicate when its upper limit of measurement is exceeded, with the mention of the value of the upper limit e.g. “result > 2 mg/L”.
The instrument shall fulfill the requirements of this Specification for the complete specified measuring range.
(2) Masking of low results
A masking function may be required which indicates 0.00 mg/L for measured mass concentrations equal to or less than a given value.
This masking function shall be deactivated in the metrological test mode.
(3) Scale interval
For the indication of the result, the scale interval shall be 0.01 mg/L in the measuring mode.
A measured value with three decimal places shall be truncated to two decimal places (e.g. a measured value of 0.427 mg/L is truncated to 0.42 mg/L).
In the metrological test mode, the EBA shall display the result with a scale interval equal to 0.001 mg/L. This scale interval shall be used for metrological tests.
Read Full notification: https://consumeraffairs.nic.in/sites/default/files/file-uploads/latestnews/Draft_Rule_Breath_Analyser.pdf