India is one of the largest producers of pharmaceutical products and a leading player in the global generics market, exporting nearly 50% of its production. The turnover of Indian pharmaceutical industry was estimated at INR 2,04,627.1 crore in FY 2015-16.
The Indian pharmaceutical industry has witnessed a robust growth in recent years growing from INR 177,734 crore in FY 2014-15 to INR 204,627 crore in FY 2015-16, registering a growth of 29% as compared to the growth of 12% from INR 158,671 crore during FY 2013-14.
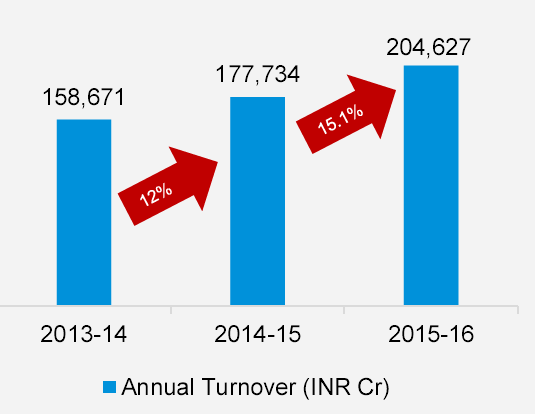
Annual Turnover
In FY 201 5-16, the exports of Drugs, Pharmaceuticals and Fine Chemicals was INR 1,06,212.4 crore. In the generics market, India exports 20% of global generics, making it the largest provider of generic medicines globally.
Policy Initiatives & Investments
FDI Policy:
- 100% FDI has been allowed through automatic route for Greenfield pharmaceuticals projects
- For Brownfield pharmaceuticals projects, FDI has been allowed up to 74% through automatic route and beyond that through government approval.
Fiscal incentives
- To promote domestic manufacturing, inverted duty structure in Medical Device industry has been corrected
- Basic customs duty has been reduced to 2.5% along with full exemption from Special Additional Duty (SAD) on raw materials, parts and accessories for manufacture of medical devices, falling under headings 9018 to 9022 e.f. January 19, 2016.
- Basic customs duty on certain specified medical devices has been increased from 5% to 7.5% to boost domestic manufacturing
- SAD of 4% has been re-imposed for specified medical devices.
- Under the ‘Credit Linked Capital Subsidy Scheme (CLCSS)’ by Ministry of Micro, Small and Medium Enterprises for technology up-gradation, micro and small pharmaceutical companies have been provided subsidies.
Major Investments & FDI Inflows
FDI Inflows: The sector saw FDI equity inflows of USD 2.25 billion from April 2014 to March 2016.
- FDI equity inflow from Apr 2016 to Sept 2016 was USD 640.71 million.
Major foreign investments in the sector (March 2014-September 2016)
| Name of Foreign Collaborator | Country | Name of Indian Company | Amount of FDI Inflows (USDM) |
| Abbot Asia Holdings Ltd | United Kingdom | Abbott Healthcare Pvt Ltd | 447.48 |
| Mylan Group B.V, Netherlands | Netherlands | Mylan Laboratories Ltd | 372.63 |
| Hospira Pte Limited
|
Singapore | Hospira Healthcare India Pvt Ltd
|
301.61 |
| Glaxo Smithkline Pte Ltd | Singapore | Glaxo Smithkline Pharmaceuticals Ltd | 228.39 |
| Jubilant Pharma Limited | Singapore | Jubilant Generics limited | 174.07 |
| Sanofi Pasteur Merieux | France | Shantha Biotecnics Limited | 123.82 |
| Fresenius Kabi (Singapore) Pte Ltd | Singapore & Germany | Fresenius Kabi Oncology Ltd | 118.25 |
| Bluewater Investment Ltd | Mauritius | Aptuit Laurus Private Ltd. | 63.49 |
| Meiji Seika Pharma Co Ltd | Japan | Medreich Ltd | 55.48 |
| Dashtag | United Kingdom | Fulford (India) Limited | 29.89 |
| Year | No. of units benefitted | Subsidy released under CLCSS |
| 2014-15 | 110 | 967.00 |
| 2015-16(as of Feb 2016) | 55 | 489.00 |
- FDI equity inflow from Apr 2016 to Sept 2016 was USD 640. 71 million
Cluster Development Programme for Pharma Sector (CDP-PS)
Launched on June 17, 2015, the scheme is being implemented on a Public Private Partnership format through a one time grant-in-aid, which will be released in phases for creation of Common Facility Centers (CFC).
Infrastructure Development
Indian Drugs and Pharmaceuticals Limited (IDPL), a Central Public Sector Enterprise under Department of Pharmaceuticals, has modernized the tablet manufacturing section of its Gurgaon Plant, which was commissioned with an investment of INR 3 crore. This has enabled the PSU to mass manufacture new products in the field of diabetes, oncology, nephrology and cardiology at affordable prices.
Other Initiatives
- As on December 15 ,2016, ceiling price of 853 formulations are under price control. The fixation of ceiling prices has resulted in a total saving of INR 2547 crore since May 2014.
- 683 Jan Aushadi stores are operational , as on December 31, 2016, to provide generic medicines to masses at cheaper price.
Ease of Doing Business initiatives
- Pharma Jan Samadhan, a customer grievances redressal system was launched in March, 2015, to address consumer complaints.
Around 820 cases of overcharging involving INR 3992 crore is currently being processed.
- Pharma Data Bank, an integrated pharmaceutical database management system was launched on June 25, 2015, to facilitate online filling of mandatory returns as prescribed in Drugs (Prices Control) Order, 2013. The database also provides a facility for submitting Form-I application for price approval of ‘new drug’ under DPCO, 2013.
- Pharma Sahi Daam, a mobile application launched in August 2016, provides real-time information to consumers on prices of Scheduled/Non-scheduled medicines.
Skill Development
To keep pace with the growing demand for highly-skilled R&D professionals the following skill development initiatives have been undertaken:
Transformation of National Institutes of Pharmaceutical Education & Research (NIPERS) as Innovation hubs
- 11 NIPERs were approved till 2015. 3 new NIPERs at Chhatisgarh, Maharashtra and Rajasthan were announced in Budget 2016-17.
- In 2015-16, INR 95.63 crore was disbursed for AICTE issued an Advisory on honouring NIPER Degrees by all
- AICTE Institutions.




