Ministry of Finance
CBIC proactively put in place a COVID Response Plan (CRP) for speedy clearances of COVID-19 vaccines at all major airports
Posted On: 15 JUL 2021
Recognizing that efficient and expeditious release of temperature-sensitive vaccines would be a critical requirement in the collective fight against the COVID-19 pandemic, CBIC has proactively put in place a COVID Response Plan (CRP) (Annexed herewith) for speedy clearances of COVID-19 vaccines at all major airports.
CRP provides for setting up of wherein a COVID-19 Vaccine Response Team (CVRT) at each Air Cargo/Courier Terminal. The CVRT will function as a single point of contact for all the clearance related to COVID-19 vaccine shipments and coordinate among the concerned stakeholders to ensure that vaccines are given instant delivery upon arrival. For this, the CVRT will develop a SoP (covering Customs, local PGA and other stakeholders) and sensitize traders on the requirements for instant release of vaccines.
Additionally, CBIC has enabled the import/export of vaccines in relation to COVID -19 through Courier, by issuing the Courier Imports and Exports (Electronic Declaration and Processing) Amendment Regulations, 2020. The Courier Regulations earlier had certain limits on the value of goods that could be brought in through courier, whereas the amended regulations allow the import and export of COVID vaccines through courier without any value limit.
As the vaccines would be transported through special containers equipped with temperature monitoring and tracking devices, provision has also been made for their duty-free temporary admission.
CBIC would closely monitor the vaccine logistics to ensure their seamless movement at the borders and address any challenges that may arise in this regard.
The COVID-19 pandemic has posed unprecedented challenges disrupting global supply chains and slowing down economic activity. CBIC had acted with agility in tackling the crisis by introducing simplified customs procedures, reducing the scale of interventions, increasing the use of automation and instituting staff health protocols.
****
(Release ID: 1735927)
CBIC’S CRP for Instant Customs Clearances of Covid-19 Vaccine
The Covid pandemic has posed a serious challenge for the health and well being of people around the world. The good news is that vaccines have been developed in various parts of the globe to help protect people and prevent the spread of the virus. The challenge for Customs the world over is to ensure that the vaccine is made available to the medical communities for use at the earliest after its import or to facilitate its export to a needy country in the shortest time possible. CBIC is prepared to meet this challenge.
Preparing for the imminent issue of authorizations for the use of vaccines for COVID-19, the CBIC has put in place a 3 step Customs Response Plan (or CRP) for instant Customs clearance of such vaccines when imported into the country.
CBIC’s 3 Step Response Plan for instant Customs clearance of COVID-19 vaccines

The challenge stems from the fact that Logistics Service Providers (LSPs) worldwide are scrambling to rapidly establish medical supply chains to deliver more than ten billion doses of vaccines. While domestic production of vaccines is being strongly encouraged and is expected to be delivered on target, the expectation is the country would also require importing the vaccines. The CBIC’s CRP aims at prompt/instant clearance of the imported vaccines immediately on their arrival or to facilitate the export of the vaccines without delay. The CRP is based on the understanding that any delay in Customs clearance would only damage the vaccine, in view of its special requirement of storage at very low temperatures. Also, CBIC is acutely conscious of the importance of the vaccines reaching the users without any loss of time.
The CRP builds upon the experience of the CBIC when, in the early days of the pandemic, as demand from medical supplies, PPEs etc. surged, significant logistics and other challenges associated with sourcing these supplies from other countries were faced by Government and others. This situation called for urgent strategizing and response which saw the Customs officers across all ports and airports of the country working 24×7 side by side the logistic providers and other stakeholders thereby ensuring there was no hold up in making available these supplies to the citizens. The Customs officers also successfully carried out their mandated task to facilitate export of essential medical and other supplies to other countries.
The CBIC’s CRP takes into account the extant Customs clearance process especially the role and responsibility of the importer/ Customs Brokers and the stakeholders in the logistics supply chain. Due attention has also been given to the finding of WHO that, the arrival of vaccines, syringes, safety boxes and other immunization supplies in the country, their subsequent clearance through Customs and their transport to the primary vaccine store are the most critical stages in a vaccine shipment; and experience shows that this is often the time when mistakes are made, and delays occur. Moreover, it is possible that the vaccines are imported/exported by those who may not be familiar with the Customs clearance process and this could possible lead to incomplete documentation, which may delay the clearance.
Thus, the CBIC has identified the areas wherein focused attention would be required to make sure that there is no delay in the Customs clearance of the imported vaccines, as follows:
Logistics processes: The logistics processes in the context of Customs clearance of imported goods typically involve the airlines or authorized courier carrying the imported goods, the ground handling agency for moving the goods to the warehouse, the custodian who receives the imported goods, presents them to the Customs, and then gives physical delivery of the goods thereby enabling their transport to the destination. There are thus many stakeholders involved and timely Customs clearance hinges on their working together in a coordinated and timely manner.
Regulatory processes: Apart from the Customs, the import of vaccine also requires authorisations from CDSCO to ensure it meets the safety and quality regulations. (More details available at:https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/biologicals/1CDSCOGuidanceForIndustry.pdf). The delay in seeking authorization or presenting the same to Customs, has the potential to delay the Customs clearance.
Capacity building of staff: Ensuring consistent temperature management of the deep frozen vaccine in a way that avoids damage to the shipments can be complicated, particularly on the volumes envisaged. LSP, officers of Customs and PGAs would need to be well conversant in the handling/clearance of such vaccines so that these are promptly cleared without getting spoilt.
Assessment of existing handling capacities: Existing cold chain storage facilities at Air Cargo Complexes (ACC) may not be equipped to handle the volumes envisaged from import/export or the intense temperature control requirements required for the vaccine. Accordingly, adequacy of infrastructure needs to be evaluated, keeping in mind the pharmacological properties of different vaccines likely to be imported/exported. Ideally, the vaccines must be moved out of the ACCs after Customs clearance, as early as possible, upon their arrival.
Limitations of working hours and non-synchronized timings between LSP, PGAs and Customs: Customs authorities presently offer a request-based facility for 24×7 clearance. However, uptake of the initiative is limited due to working hours of LSPs and PGAs, including weekends and holidays.
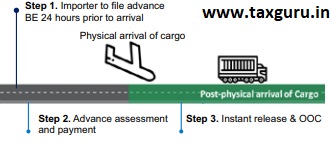
Another very important innovation is that for the imported vaccines, the CBIC has focused on shifting the processes that are ordinarily done post the arrival of the imported goods to stages either before their arrival or post their Customs clearance i.e., after the importer has already taken delivery of the goods. The completion of certain processes in advance of the arrival of the vaccines would, of course, require support of the stakeholders. The CBIC is confident that this approach would result in the vaccines becoming available to the users instantly upon their arrival.
Thus, the CBIC’s CRP provides a one-stop solution for fast tracked Customs clearance of the vaccines imported for COVID-19 (and for export of such vaccines, when needed). As regards the export of the vaccine, the same is covered by Government Policy
Indicative process flow for Instant Customs Clearance via CRP
As regards the export of the vaccine that is covered by Government Policy Formation of a COVID-19 Vaccine Response Team: COVID-19 Vaccine Response Team (CVRT) shall be established at Air Cargo Complexes in five locations, i.e., Bangalore, Chennai, Delhi, Hyderabad, and Mumbai. This team will comprise of Customs officers at the level of Assistant Commissioner / Deputy Commissioner (AC/DC), Officers from concerned PGAs, Representatives from Customs Brokers Association and the concerned LSPs. CVRT teams shall be the single point of contact for all the clearance related to vaccine shipments. The CVRT will be responsible for close coordination among the concerned stakeholders for handholding the importers/exporters and ensure seamless Exim procedures for the vaccine. Officers of the CVRT have distinct designated roles vis-à-vis import/export of consignments. Contact details of Zonal CVRT and an escalation matrix is provided at Annexure 1. Other responsibilities of the CVRT include:
All Customs locations which are expected to handle vaccines shall institute a COVID-19 Vaccine Response Team (CVRT) for instant clearance.
1. Development of SoP: Develop Localised Standard Operating Procedure, standardize the Exim processes associated with the vaccine shipment, including steps required for logistics handling. The SoP shall cover:
CVRT will develop a SoP (for Customs, local PGA and other stakeholders) and sensitize identified traders on the requirements for instant release of vaccines.
a. Common helpdesk with contact email/ telephone for these import / export shipments.
b. Verification of standard approvals necessary for clearance. (Includes required certificates from Drug Controller.)
c. Handholding of the Importer/Exporter, if required.
d. Mechanism for Importer to notify the shipment details and file Advance Bill of Entry for instant clearance.
e. Standardised process for handling the vaccine on arrival, including the places designated for storage and handling.
f. Simplified and instant process for amendments of Customs clearance documents and other special procedures, if required.
A Public Notice shall be issued on the above to ensure widespread awareness of the SoP among importers and the larger trading community. These measures are necessary to ensure minimum variance in process duration, which can pose to be a challenge given the consistent cooling requirements.
The SoP should be published post liaison with local officers of CDSCO to understand the planned measures to facilitate vaccine imports and associated documents, including NOCs.
2. Sensitization: CVRT shall reach out to Custom Brokers (CBs) and LSPs and potential importers/exporters in their jurisdiction to
increase awareness of measures undertaken by CBIC for seamless clearance of vaccine shipments. They shall sensitize the CBs and the LSPs of the requirements of the SoP.
3. Infrastructure Assessment: CVRT will assess/inspect storage site(s) and determine holding capacity for storage on arrival pending issuance of Out-Of-Charge (OOC) / LET Export Order (LEO), at each Customs location. CVRT will subsequently institutionalize a mechanism to monitor capacity utilization of storage site against advance BE/SB filed and OOC / LEO issued. CVRT will also validate adequacy of provisions for handling equipment for Customs and PGA officers, including gloves for handling vaccine stored in UCC. Necessary training will be imparted to Customs and PGA officers to handle the consignment in consultation with the LSP.
4. Intimation for Instant Release: The CVRT shall have list of importers/exporter approved by CDSCO for vaccine import. However, there will be an additional mechanism for intimation by the importer/exporter requiring instant release and to do necessary check for the availability of approvals from CDSCO and other requirements needed by logistics operators in advance. Prior to arrival/export of vaccines, importer/exporter may provide intimation on dedicated email ID regarding the scheduled arrival/export of the consignments. The Customs shall intimate the CB, LSP and PGA with regarding advance filing of documents and necessary arrival/ export details for stakeholders to plan subsequent logistics movement.
Importers are encouraged to ensure timely filing of their BE and applicable NOC to enable Customs to complete all clearance processes in advance of arrival of the vaccines.
5. Advance filing of BE and NOCs: Advance error free filing of Bill of Entry can ensure instant clearance on arrival of the vaccine.
a. Importers are advised to file BE 24 hours in advance
b. While filing BE, ensure that details of manufacturer, correct HS code, End Use details and CDSCO related forms are correctly mentioned in the BE to enable higher facilitation.
c. Bond requirements for containers of durable nature may be included, if required.
This enables CVRT Team to track the consignment and enable instant clearance within two hours of arrival provided requisite documentation including BE and required NoCs are filed in advance.
6. Release on Arrival: Considering the sensitive nature of the vaccines, Customs Officers (viz. IFO) posted in the Shed, will allow Instant release of the vaccines on payment of duties, if applicable, on production of following documents:
CVRTs shall develop a joint working mechanism for 24×7 clearance in consultation with LSP and PGA.
I. Importer Intimation for Instant Release
II. Advance Air Way Bill (AWB) Filed for this consignment
III. Provisional NOC from Drug Controller
IV. Summary Details of durable Containers on temporary import.
V. Bill of Lading
Examination of such shipments would be limited to cases of specific intelligence or information and only with the prior permission of the concerned AC/DC. In case of odd hours, the documentation related to Out of Charge, would be undertaken in the working hours, on the next day.
7. Planning for 24×7 working across logistics players, PGAs and Customs: Each CVRT shall establish protocols for 24×7 clearance by Customs and PGAs in vaccine transport hubs to clear the consignments on arrival or on bond. Necessary, arrangements are to be made for vaccine regulatory and logistics movements to take place on weekends and holidays.
CVRTs shall plan for their schedules to permit 24×7 working, including on holidays, to ensure seamless movement of vaccine consignments. The jurisdictional Commissioner may necessarily augment manpower, to meet this requirement. The CVRT would also maintain a time schedule for officers to be present at Customs stations for facilitation. Specific duty schedule should be assigned for officers who would be deputed for the facilitation of vaccine shipments. A joint working protocol shall be established along with LSPs, Custodian and PGAs to address the requirement of working beyond the regular hours
8. Classification for Vaccines: The vaccines made exclusively for COVID-19 would be classified under CTH 30022019.
9. ICETRAK: Stakeholders can check the live status of their BE/SB using the ICETRAK mobile application and validate the gate pass/ BE/SB copies with QR code scanning.
Screenshots of ICETRAK application
10. Provision for re-export of vaccine containers: The temporary import of durable containers used for vaccine transport which may need to be re-exported within six months from the date of import shall be handled in accordance with provisions of Notification No. 104/94-Customs, dated 16.03.1994 (as amended.) In this regard, CBIC has also issued Circular No. 51/2020-Cus., dated 20.11.2020 to clarify the procedure for availing Custom duty exemption on temporary import of durable containers.
11. e-Sealing facility for exports: Vaccine manufacturers may be encouraged to avail self-sealing permission to enable seamless movement of export vaccine shipments.
12. Courier facility: CBIC has also issued notification vide which COVID-19 vaccines can also be imported/exported through courier mode.
13. Further References:
Clearances Related Information : www.cbic.gov.in or www.icegate.gov.in
https://www.icegate.gov.in/COVID19-Measures.html
COVID Contact: https://icegate.gov.in/coronavirus-tradehelp/
CDSCO related Information: www.cdsco.gov.in