The Government of India, through the Legal Metrology Division of the Ministry of Consumer Affairs, has issued draft rules for non-invasive automated sphygmomanometers, inviting stakeholder feedback. The proposed rules, framed under the Legal Metrology Act, 2009, are published on the department’s website for public review. Stakeholders, including state metrology departments, industry bodies, and voluntary consumer organizations, are requested to submit their comments by 30th December 2024. The rules outline technical and metrological requirements for these medical devices, specifying accuracy levels, operational ranges, and design parameters for components such as cuffs, bladders, and pressure systems. They also set standards for error margins under various conditions and requirements for reusable equipment. The proposed regulations aim to ensure safety, performance, and consistency in blood pressure measurement devices, applicable from 1st July 2025. Feedback can be sent to the provided email addresses to finalize the framework.
I-9/13/2024-W&M
Government of India
Ministry of Consumer Affairs, Food & Public Distribution
Department of Consumer Affairs
Legal Metrology Division
Krishi Bhawan, New Delhi-01
Dated: 29.11.2024
Subject– Draft Rules for Non-invasive automated sphygmomanometer for comments from stakeholders – reg.
Undersigned is directed to refer to the above mentioned subject and to state that the Draft Rules for Noninvasive automated sphygmomanometer are placed in the website of the Department www.consumeraffairs.nic.in for seeking comments from all stakeholders by 30.12.2024. The comments may be sent to email-ID: dirwm-ca@nic.in/ mk.naik72@gov.in.
(Ashutosh Agarwal)
Director (Legal Metrology)
Ph: 011-23389489
Email: dirwm-ca@nic.in To:
All concerned (State Legal Metrology Departments, VCOs, Industries and Industry Associations)
[TO BE PUBLISHED IN THE GAZETTE OF INDIA, EXTRAORDINARY, PART II SECTION 3 SUB-SECTION (i)]
GOVERNMENT OF INDIA
MINISTRY OF CONSUMER AFFAIRS, FOODAND PUBLIC DISTRIBUTION
(DEPARTMENT OF CONSUMER AFFAIRS)
NOTIFICATION
New Delhi, the …………………….. 2024
GSR……. (E). – In exercise of the powers conferred by sub-section (1) read with clauses (c), (f),
(h), (i) and (s) of sub-section (1) of section 52 of the Legal Metrology Act 2009, (1 of 2010), the Central Government hereby makes the following rules, namely:-
1. Short title and commencement
(1) These rules may be called the Legal Metrology (General) Second Amendment Rules, 2024.
(2) They shall come into force on 1st July, 2025.
2. In the Legal Metrology (General) Rules, 2011, (herein after referred to as the said rules), under the Eight Schedule Part VII B and the entries made thereunder, the following shall be substituted, namely.-
PART VII B
Non-Invasive Automated Sphygmomanometers
SPECIFICATIONS FOR MEASURING INSTRUMENTS
Part 1: Metrological and Technical requirements
1. Scope
This Part specifies general, performance, efficiency and mechanical safety requirements for non-invasive automated sphygmomanometers and their accessories which, by means of an inflatable cuff, are used for the non-invasive measurement of arterial blood pressure.
This specification only applies to devices measuring at the arm, the wrist or the thigh.
2. Terminology
(1) auscultatory method,-
The method whereby sounds (known as Korotkoff sounds) are heard or detected (e.g. by a microphone) over an occluded artery as the occluding pressure is slowly released, the appearance of sounds coinciding with the systolic blood pressure and the disappearance of sounds with the diastolic blood pressure
(2) bladder,-
the inflatable component of the cuff
(3) cuff,-
Component of the non-invasive automated sphygmomanometer, comprising a bladder and a sleeve, which is wrapped around the limb of the patient
Note: A cuff might comprise a bladder and an inelastic part that encloses the bladder, or
have an integral bladder (i.e. the cuff including the bladder are fixed together or are one piece).
(4) deflation valve,-
Valve for controlled exhaust of the pneumatic system during measurement
(5) diastolic blood pressure (value),-
Minimum value of the arterial blood pressure as a result of relaxation of the systemic
ventricle
Note: Because of hydrostatic effects, this value should be measured with the cuff at the heart level.
(6) manometer,-
Instrument used to measure and display pressure
(7) mean arterial blood pressure (value),-
Value of the integral of one cycle of the blood pressure curve divided by the time of one
heart beat period
Note: Because of hydrostatic effects, this value should be measured with the cuff at the heart level.
The calculation of the mean arterial blood pressure using only the systolic and diastolic blood pressure values is not recommended.
(8) non-invasive automated sphygmomanometer,-
Medical measuring instrument used for the intermittent non-invasive estimation of the blood pressure by utilising an inflatable cuff, a pressure transducer, a valve for deflation, and/or displays used in conjunction with automated methods for estimating blood pressure. Hereafter referred to as “sphygmomanometer” in this specification
(9) non-invasive blood pressure measurement,-
Indirect measurement of the arterial blood pressure without arterial puncture
(10) oscillometric method,-
Method that estimates systolic, diastolic and mean arterial pressures during the slow inflation or deflation of an occluding cuff at the brachial artery
Note: During the inflation and deflation of the cuff, small pressure changes (oscillations) occur in the cuff as a result of the arterial blood pressure pulses. These oscillations are detected and stored together with the corresponding cuff pressure values in the measurement system.
With these stored values the systolic, diastolic and mean arterial blood pressure values can be mathematically derived using an appropriate algorithm.
(11) patient simulator,-
Device for simulating the oscillometric cuff pulses and/or auscultatory sounds during
inflation and deflation
Note: This device is not used for testing measurement accuracy but is required in assessing stability of performance.
(12) pneumatic system,-
System that includes all pressurised and pressure-controlling parts such as cuff, tubing,
connectors, valves, transducer and pump
(13) rapid exhaust valve,-
Valve for rapidly exhausting the pneumatic system
(14) sleeve
Essentially inelastic part of the cuff that encloses the bladder
(15) systolic blood pressure (value),-
Maximum value of the arterial blood pressure as a result of the contraction of the systemic
ventricle
Note: Because of hydrostatic effects, this value should be measured with the cuff at the
heart level.
(16) tamper proofing,-
Means of preventing the user from gaining easy access to the measuring mechanism of the device
(17) zero adjustment of a measuring system,-
Procedure that corrects a deviation of the pressure reading to 0.0 kPa (0 mmHg) at atmospheric pressure (gauge pressure: 0 kPa (0 mmHg))
3. Description of the category of instrument.-
The basic components of a sphygmomanometer are a cuff that can be wrapped around a patient’s limb, a system for applying and releasing pressure to the bladder in the cuff, and a means of measuring and displaying blood pressure values automatically.
Note 1: Specific device types included in this category are: sphygmomanometers for self-measurement, blood pressure monitors and multi-parameter patient monitors used for home healthcare, or public use.
Note 2: Components of a sphygmomanometer include: manometer, cuff, valve for deflation (often in combination with the valve for rapidly exhausting the pneumatic system), pump for inflation of the bladder, and connection tubing.
4. Units of measurement.-
The blood pressure shall be indicated either in kilopascals (kPa) or in millimetres of mercury (mmHg).
5. Metrological requirements.-
(1) Maximum permissible errors of the cuff pressure indication under ambient conditions,-
For any set of conditions within the ambient temperature range from 10 °C to 40 °C and the relative humidity range from 15 % to 85 %, both for increasing and for decreasing pressure, the maximum permissible error for the measurement of the cuff pressure at any point of the measurement range shall be ±0.4 kPa (±3 mmHg) or ±2 % of the reading, whichever is greater.
(2) Maximum permissible errors of the blood pressure measurement as determined by clinical investigation,-
The following maximum permissible errors shall apply for the sphygmomanometer:
(i) maximum mean error of measurement: ±0.7 kPa (±5 mmHg);
(ii) maximum experimental standard deviation: 1.1 kPa (8 mmHg).
(3) Maximum permissible errors of the cuff pressure indication under storage conditions,-
The sphygmomanometer shall maintain the requirements specified in this specification after storage for 24 h at a low temperature of -5 °C, followed by additional storage for 24 h at a high temperature of 50 °C and at a relative humidity of 85 % (non-condensing).
(4) Blood pressure measurement range,-
The sphygmomanometer shall be capable of indicating diastolic blood pressure over at least the range from 2.7 kPa (20 mmHg) to 8.0 kPa (60 mmHg) in neonatal mode, and 5.3 kPa (40 mmHg) to 17.3 kPa (130 mmHg) otherwise.
The sphygmomanometer shall be capable of indicating systolic blood pressure over at least the range from 5.3 kPa (40 mmHg) to 14.7 kPa (110 mmHg) in neonatal mode, and 8.0 kPa (60 mmHg) to 30.7 kPa (230 mmHg) otherwise.
(5) Repeatability of blood pressure indication,-
For any set of conditions within the ambient temperature range from 10 °C to 40 °C and the relative humidity in the range from 15 % to 85 %, the experimental standard deviation of the blood pressure indication of the sphygmomanometer shall not exceed 0.4 kPa (3 mmHg).
6. Technical requirements.-
(1) General,-
Equipment, or parts thereof, using materials or designs different from those detailed in this specification shall be accepted if it can be demonstrated that an equivalent degree of safety and performance is obtained.
(2) Technical requirements for the cuff and bladder,-
The cuff shall contain or incorporate a bladder. The cuff shall be designed and marked (i.e. using permitted circumference indicators) to ensure and restrict the use of the appropriate cuff size corresponding to a given limb circumference.
The bladder length should be approximately 0.80 × the circumference of the limb at the midpoint of the intended range of the cuff. The width of the bladder should be at least 0.40 × the circumference of the limb at the midpoint of the intended range of the cuff.
For reusable cuffs the manufacturer shall indicate the method for cleaning in the accompanying documents.
(3) Effect of voltage variations of the power source,-
(i) Internal electrical power source
Changes in the voltage within the working range specified by the manufacturer shall not influence the cuff pressure indication.
Outside this working range no cuff pressure indication and no result of the blood pressure measurement shall be displayed.
(ii) External electrical power source
Changes in the voltage within the working range specified by the manufacturer shall not influence the cuff pressure indication.
Outside the working range specified by the manufacturer, no cuff pressure indication and no result of the blood pressure measurement shall be displayed.
(4) Pneumatic system,-
(i) Air leakage
Air leakage shall not exceed a pressure drop of 0.8 kPa/min (6 mmHg/min).
(ii) Pressure reduction rate of devices using the auscultatory method
The pressure reducing system for manually operated and automated deflation valves shall be capable of maintaining a deflation rate of 0.3 kPa/s to 0.4 kPa/s (2 mmHg/s to 3 mmHg/s) within the target range of systolic and diastolic blood pressure. For devices which control the pressure reduction as a function of the pulse rate, a deflation rate of 0.3 kPa/pulse to 0.4 kPa/pulse (2 mmHg/pulse to 3 mmHg/pulse) shall be maintained.
Note: Manually operated deflation valves should be easily adjustable to these values.
(iii) Rapid exhaust
During the rapid exhaust of the pneumatic system, with the valve fully opened, the time for the pressure reduction from 34.7 kPa to 2.0 kPa (260 mmHg to 15 mmHg) shall not exceed 10 s.
For the sphygmomanometer having the capability to measure in a neonatal/infant mode, the time for the pressure reduction from 20.0 kPa to 0.7 kPa (150 mmHg to 5 mmHg) during the rapid exhaust of the pneumatic system with the valve fully opened shall not exceed 5 s.
(iv) Zero adjustment of a measuring system
The sphygmomanometer shall be capable of automatic zero adjustment. The zero adjustment shall be carried out at appropriate intervals, at least when the device is powered on. After a zero adjustment, the device shall keep the indication of a gauge pressure of 0.0 kPa (0 mmHg).
The sphygmomanometer shall repeat a zero adjustment or shall be switched off automatically when the output of the pressure transducer drifts one scale interval (0.1 kPa or 1 mmHg) or more.
(v) Manometer test mode
The sphygmomanometer shall have a manometer test mode that permits static pressure measurement over at least the nominal blood pressure indication range. This mode shall not be available in normal use, but restricted to service / test personnel.
When the sphygmomanometer is put into the test mode, all air outlets shall be closed.
The manufacturer shall confirm that the test results obtained in sub-paragraph (1) of paragraph 5 and clause (iv) of sub-paragraph (4) of paragraph 6 of this Part are identical to the results in the normal use mode.
(vi) Maximum time for which the cuff is inflated
The total time for which the pressure exceeds 2.0 kPa (15 mmHg) shall be no longer than 180 s in the case of adult patients. The total time for which the pressure exceeds 0.7 kPa (5 mmHg) shall be no longer than 90 s in the case of neonatal/infant patients.
(5) Electromagnetic compatibility,-
(i) Immunity
The following requirements apply:
(a) electrical and/or electromagnetic interferences shall not lead to degradations in the cuff pressure indication, i.e. the maximum permissible error for the measurement of the cuff pressure shall be ±0.4 kPa (±3 mmHg) or ±2 % of the reading, whichever is greater; or
(b) if electrical and/or electromagnetic interferences lead to an abnormality, the abnormality shall be clearly indicated and it shall be possible to restore normal operation within 30 s after cessation of the electromagnetic disturbance.
(ii) Electrosurgery interference recovery
If a sphygmomanometer is intended to be used together with HF surgical equipment, it shall return to the previous operating mode within 10 s after exposure to the field produced by the HF surgical equipment, without loss of any stored data.
(6) Durability,-
The change in the cuff pressure indication shall not be greater than 0.4 kPa (3 mmHg) throughout the pressure range after 10 000 simulated measurement cycles.
(7) Technical requirements for the pressure indicating device,-
(i) Nominal range and measurement range of the cuff pressure measurement
The nominal range for the cuff pressure measurement shall be specified by the manufacturer. The measurement range of the cuff pressure shall be equal to the nominal range. Values of blood pressure measurement results outside the nominal range of cuff pressure shall be clearly indicated as out of range.
Testing shall be carried out by visual inspection.
(ii) Digital indication
The digital scale interval shall be 0.1 kPa (1 mmHg).
If the measured value of a parameter is to be indicated on more than one display, all the displays shall indicate the same numerical value.
Measured numerical values on the display(s), and the symbols defining the units of measurement shall be arranged in such a way so as to avoid misinterpretation.
Numbers and characters should be clearly legible.
Testing shall be carried out by visual inspection.
(iii) Technical requirements for the display
The display shall be designed and arranged so that all information can be read and easily
recognised.
If abbreviations are used on the display they shall be as follows:
(a) “S” or “SYS”: systolic blood pressure (value);
(b) “D” or “DIA”: diastolic blood pressure (value);
(c) “M” or “MAP”: mean arterial blood pressure (value)
Single letter abbreviations shall be positioned in such a way to avoid confusion with SI units.
Testing shall be carried out by visual inspection.
(8) Signal input and output ports,-
The construction of the signal input and output ports (excluding internal interfaces, e.g. microphone signal input) relevant to the non-invasive blood pressure measurement shall ensure that incorrectly fitted or defective accessories shall not result in erroneous indication of cuff pressure or erroneous indication of blood pressure.
Note: An error message or a blank display is sufficient.
(9) Safety requirements,-
(i) Aborting a measurement
It shall be possible to abort any blood pressure measurement at any time by single key operation and this shall lead to a rapid exhaust.
(ii) Unauthorised access and tamper proofing
All controls which affect accuracy shall be sealed against unauthorised access.
Tamper proofing of the instrument shall be achieved by requiring the use of a special tool or breaking a seal.
Testing shall be carried out by visual inspection.
It shall be clear to an operator if tampering or unauthorised access has occurred.
(iii) Tubing connectors
Luer lock and Luer slip connectors shall not be used on sphygmomanometers so as to avoid any risk of connecting the output of the sphygmomanometer to intravascular fluid systems as air might inadvertently be pumped into a blood vessel.
(iv) Electrical safety
Automated sphygmomanometers shall comply with the relevant national safety regulations.
(10) Resistance to vibration and shock,-
The sphygmomanometer or its parts not intended for use during patient transport outside a healthcare facility shall have adequate mechanical strength when subjected to mechanical stress caused by normal use, pushing, impact, dropping, and rough handling. A fixed (e.g. wall mounted) sphygmomanometer is exempt from the requirements of this sub clause.
After the test for the resistance to vibration and shock, the sphygmomanometer shall comply with the requirements of sub-paragraph (1) of paragraph 5 of this part but only at a temperature of 20 °C ± 5 °C and at ambient humidity.
(11) Durability of markings,-
The markings shall be removable only with a tool or by appreciable force and shall be sufficiently durable to remain clearly legible during the expected service life of the sphygmomanometer. In considering the durability of the markings, the effect of normal use shall be taken into account.
7. Metrological controls.-
(1) Model approval,-
At least three samples of a new type of sphygmomanometer shall be tested.
The tests to verify conformity to metrological and technical requirements shall be carried out according to Part 2 of this specification.
(2) Verification,-
At verification, testing shall be conducted at any set of climatic conditions within the temperature range from 10 °C to 40 °C and the relative humidity range from 15 % to 85 %. A climatic chamber is not required.
The requirements of sub-paragraph (1) of paragraph 5, sub-paragraph (1) of paragraph 5 and clause (i) of sub-paragraph (1) of paragraph 6(4) of this Part shall be fulfilled.
(3) Sealing,-
Metrological control marks shall be put on seals. These seals shall prevent, without destruction of the control marks:
(i) in the case of patient-monitors in which the sphygmomanometer is one part of a system: the manipulation of the metrologically relevant parts for measuring blood pressure;
(ii) in the case of all other sphygmomanometers: the opening of the casing.
If the construction of the instrument guarantees security against any interference, the metrological control marks or the security marks may be attached in the form of labels.
All seals shall be accessible without using a tool.
(4) Marking of the device,-
(i) Markings required on the indicating device
The indicating device of the sphygmomanometer shall be marked with the following information:
(a) name and/or trademark of the manufacturer;
(b) type of sphygmomanometer;
(c) units of measurement (kPa/mmHg), positioned close to the displayed values;
(d) measurement range;
(e) model approval number ;
(f) serial number;
(g) year of fabrication;
(h) country of origin.
Testing shall be carried out by visual inspection
(ii) Markings required on the cuff
The cuff of the sphygmomanometer shall be marked with the following information:
(a) limb circumference for which it is appropriate;
(b) marking of the limb circumference indication range;
(c) centre of the bladder, indicating the correct position for the cuff over the artery.
Testing shall be carried out by visual inspection.
For sphygmomanometers applied to the wrist, the marks required in clause (i) of sub- paragraph(4) of paragraph 7 and clause (ii) of sub-paragraph(4) of paragraph 7 of this Part can be positioned on the indicating device or on the cuff.
For sphygmomanometers used for home healthcare environment, the sales packaging shall display information needed by the end user including, as a minimum:
(d) the operating and storage temperature and humidity ranges;
(e) any special requirements for a battery-powered sphygmomanometer. Sphygmomanometers for public use which are intended for self-use in public areas shall be marked with the following:
(f) precautions for use, including a statement concerning the need to consult a physician for interpretation of blood pressure measurements;
(g adequate operating instructions
(5) Manufacturer’s information,-
(i) Information supplied by the manufacturer shall comply with the specifications and requirements given in this specification.
(ii) The manufacturer’s instruction manual shall contain the following information:
(a) explanation of the operating procedures which are important for correct application (such as the selection of the appropriate cuff size, positioning of the cuff at the heart level and adjustment of the pressure reduction rate);
(b) methods for cleaning reusable cuffs;
(c) if the bladder is removable, the method for ensuring the correct repositioning of the bladder in the cuff;
(d) nature and frequency of the maintenance which is required to ensure that the device operates correctly and safely at all times;
(e) list of all components belonging to the pressure measuring system, including accessories;
(f) description of the operating principles of the blood pressure measuring device;
(g) remarks on the environmental or operational factors which may affect the performance (e.g. electromagnetic fields, arrhythmia);
(h) specification of the signal input/output port(s);
(i) specification of the rated voltage, if applicable;
(j) specification of the intended power source, if applicable;
(k) measurement range for the systolic and diastolic blood pressure measurements;
(l) measurement range of the pulse rate;
(m) operating and storage temperature and humidity ranges;
(n) any special requirements for a battery-powered automatic sphygmomanometer, e.g. safety warnings;
(o) warm-up time, if applicable;
(p) description of the meaning of the “out of range signal”;
(q) description of all symbols, abbreviations and error codes used on the instrument; and
(r) name and address of manufacturer.
Testing shall be carried out by visual inspection.
Part 2: Test Procedures
1. Test for maximum permissible errors of the cuff pressure indication.-
(1) Apparatus,-
The apparatus consists of the following:
(i) rigid metal vessel with a capacity of 500 ml ± 25 ml;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) pressure generator, e.g. ball pump (hand pump) with a deflation valve;
(iv) T-piece connectors and hoses with an overall length of no more than 600 mm;
(v) climatic chamber, non-uniformity of temperature within ±1 °C, instability of temperature within ±1 °C, non-uniformity of relative humidity within ±5 %, instability of relative humidity within ±5 %.
(2) Procedure,-
Replace the cuff with the vessel. Connect both the calibrated reference manometer and the manometer of the device to be tested by means of a T-piece connector and hoses to the pneumatic system (see Figure 1). Set the automated sphygmomanometer to the test mode according to the information provided by the manufacturer. Connect the additional pressure generator into the pressure system by means of another T-piece connector. Carry out the test in pressure steps of not more than 6.7 kPa (50 mmHg) between 0.0 kPa (0 mmHg) and the maximum pressure of the scale range.
(i) Under ambient conditions
For each of the following combinations of temperature and humidity, place the automated sphygmomanometer in the climatic chamber for at least 3 h to allow the system to reach steady conditions:
(a) 10 °C ambient temperature, 85 % relative humidity (non-condensing);
(b) 20 °C ambient temperature, 85 % relative humidity (non-condensing);
(c) 40 °C ambient temperature, 85 % relative humidity (non-condensing).
At each combination of temperature and humidity, switch on the automated sphygmomanometer before starting the test. Wait until the warm-up time (described in the instructions for use) has elapsed, carry out the measurement and switch off the automated sphygmomanometer afterwards.
(ii) Under storage conditions
Testing shall be carried out (a) under ambient conditions after the test sample has been placed unpacked for 24 h at a temperature of –5 °C and immediately afterwards for 24 h at a temperature of 50 °C in a climatic chamber. Any abnormal status shall be recorded during the testing.
Note: Integrated multi-parameter monitors may contain components which may be damaged during storage. The general temperature range has therefore been reduced compared to the requirements in Part 1, 5(1). For simplification, testing can also be carried out at a temperature of 20 °C ± 5 °C and at ambient humidity.
(3) Expression of results,-
Express the results as the differences between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer.
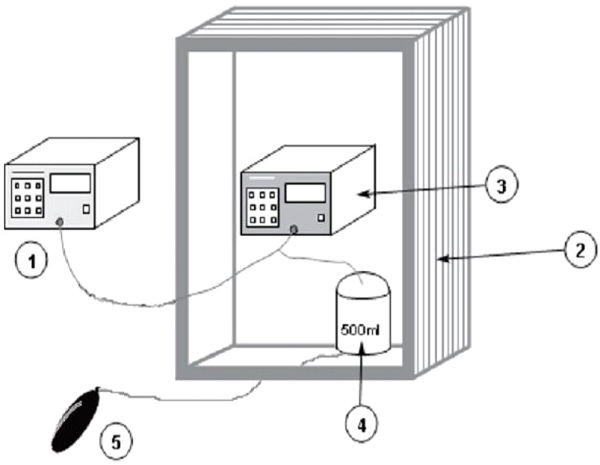
1 – Reference manometer;
2 – Climatic chamber;
3 – Device to be tested;
4 – Metal vessel;
5 – Pressure generator
Figure 1 – Measurement system for determining the error limits of the cuff pressure indication
2. Test for blood pressure measurement range.-
To comply with the requirement of sub-paragraph (4) of paragraph 5 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) Patient simulator for the auscultatory and/or oscillometric method, having an experimental standard deviation for the repeatability of not more than 0.13 kPa (1 mmHg). The generated signal values shall be approximately: systolic: 16.0 kPa (120 mmHg); diastolic: 10.7 kPa (80 mmHg); pulse rate: 70 min-1 to 80 min-1.
(2) Procedure and evaluation,-
Adjust the patient simulator to generate signals in such a way that the automated sphygmomanometer displays diastolic blood pressure values of 2.7 kPa (20 mmHg) or less and systolic blood pressure values of 14.7 kPa (110 mmHg) or more in neonatal mode, and diastolic blood pressure values of 5.3 kPa (40 mmHg) or less and systolic blood pressure values of 30.7 kPa (230 mmHg) or more otherwise.
Check by visual inspection.
3. Test for repeatability of blood pressure indication.-
To comply with the requirement of sub-paragraph (5) of paragraph 5 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) Patient simulator for the auscultatory and/or oscillometric method, having an experimental standard deviation for the repeatability of not more than 0.13 kPa (1 mmHg). The generated signal values shall be approximately: systolic: 16.0 kPa (120 mmHg);diastolic: 10.7 kPa (80 mmHg);pulse rate: 70 min-1 to 80 min-1.
(2) Procedure,-
Connect the automated sphygmomanometer with the cuff and the patient simulator, which is set to the target systolic and diastolic blood pressure values (see Figure 2).
Perform 20 consecutive measurements at any temperature in the range 10 °C to 40 °C and for any relative humidity in the range 15 % to 85 %.
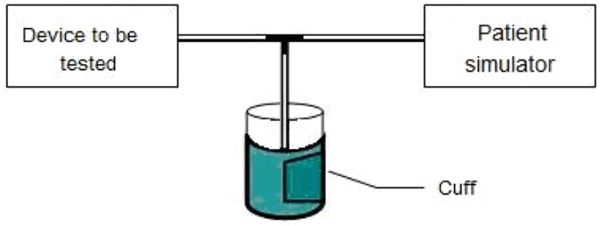
Figure 2 – Setup to test the repeatability of the blood pressure indication
Note 1: If the device is being tested for adult blood pressure measurement, the pulse rate is set at 80 min-1.
Note 2: If the device is being tested for neonatal/infant blood pressure measurement, the pulse rate is set at 120 min-1.
(3) Expression of results
The repeatability of a blood pressure indication is calculated as follows:
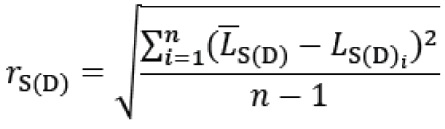
rS(D) being the display value repeatability of systolic (or diastolic) blood pressure of the device under test;
LS(D)i being the displayed systolic (or diastolic) blood pressure at the ith measurement of the device under test;
S(D) being the displayed mean of systolic (or diastolic) blood pressure of the device under test;
n number of measurements.
4. Test for effect of voltage variations of the power source.-
To comply with the requirement of sub-paragraph (3) of paragraph 6 of Part 1, the following test shall be performed.
(1) Internal electrical power source,-
(i) Apparatus
The apparatus consists of the following:
(a) adjustable direct current voltage supply;
(b) voltmeter with a maximum permissible error within 0.5 % of the measured value;
(c) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg).
(ii) Procedure
Replace the internal electrical power source of the automated sphygmomanometer with a DC voltage supply having an impedance which is equivalent to the impedance of the internal electrical power source specified by the manufacturer. Measure the variation in applied DC voltage supply with a voltmeter. Test the automated sphygmomanometer by altering the DC voltage supply in steps of 0.1 V and determine the lowest voltage limit at which the cuff pressure indication is still displayed.
Carry out this test with the maximum permissible impedance of the internal electrical power source.
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity, and at the lowest voltage limit described above increased by 0.1 V and also at the nominal voltage.
(iii) Expression of results
Express the results as the difference between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer at the lowest voltage limit increased by 0.1 V and at the nominal voltage.
(2) External electrical power source – alternating current,-
(i) Apparatus
The apparatus consists of the following:
(a) adjustable alternating current voltage supply;
(b) voltmeter with a maximum permissible error within 0.5 % of the measured value;
(c) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg).
(ii) Procedure
Connect the automated sphygmomanometer to the adjustable alternating current voltage supply. Measure the variation in AC voltage supply with the voltmeter.
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity at:
(a) the maximum rated voltage, declared by the manufacturer;
(b) the mean value of the maximum and minimum rated voltage, declared by the manufacturer;
(c) the minimum rated voltage, declared by the manufacturer.
Testing may be carried out at only one cuff pressure within the range 6.7 kPa to 33.3 kPa
(50 mmHg to 250 mmHg).
Note: The maximum rated voltage is declared by the manufacturer as well as the minimum rated voltage.
(iii) Expression of results
Express the results as the difference between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer.
(3) External electrical power source – direct current,-
(i) Apparatus
Use the apparatus listed in clause (i) of sub-paragraph (1) of paragraph 4 of this Part.
(ii) Procedure
Connect the automated sphygmomanometer to the DC voltage supply. Control the DC voltage supply by reference to a voltmeter.
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity at:
(a) the maximum rated voltage, declared by the manufacturer;
(b) the mean value of the maximum and minimum rated voltage, declared by the manufacturer;
(c) the minimum rated voltage, declared by the manufacturer.
Testing can be carried out only at one cuff pressure point within the range 6.7 kPa to 33.3 kPa (50 mmHg to 250 mmHg).
(iii) Expression of results
Express the results as the difference between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer.
(4) Voltage variations of the external electrical power source – alternating current,-
(i) Apparatus
Use the apparatus listed in clause (i) of sub-paragraph (2) of paragraph 4 of this Part.
(ii)Procedure
Connect the automated sphygmomanometer to the AC voltage supply. Measure the variation in the AC voltage supply with the voltmeter. Test the automated sphygmomanometer by altering the AC voltage supply in steps of 5 V and determine the lowest voltage limit at which the cuff pressure indication is displayed.
Outside the working range specified by the manufacturer, no cuff pressure indication shall be displayed.
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity at the lowest voltage limit increased by 5 V.
Testing can be carried out only at one cuff pressure point within the range 6.7 kPa to 33.3 kPa (50 mmHg to 250 mmHg).
(iii) Expression of results
Express the results as the difference between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer at the lowest voltage limit increased by 5 V.
(5) Voltage variations of the external electrical power source – direct current,-
(i) Apparatus
Use the apparatus listed in clause (i) of sub-paragraph (1) of paragraph 4 of this Part.
(ii) Procedure
Connect the automated sphygmomanometer to the DC voltage supply. Measure the variation in the DC voltage supply with the voltmeter.
Test the automated sphygmomanometer by altering the DC voltage supply in steps of 0.1 V and determine the lowest voltage limit at which the cuff pressure indication is displayed.
Outside the working range specified by the manufacturer, no cuff pressure indication shall be displayed.
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity at the lowest voltage limit increased by 0.1 V.
Testing can be carried out only at one cuff pressure point within the range 6.7 kPa to 33.3 kPa (50 mmHg to 250 mmHg).
(iii) Expression of results
Express the results as the difference between the cuff pressure indication of the automated sphygmomanometer to be tested and the corresponding readings of the reference manometer at the lowest voltage limit increased by 0.1 V.
5. Test for air leakage of the pneumatic system.-
To comply with the requirement of clause (i) of sub-paragraph (4) of paragraph 6 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) rigid metal cylinder of an appropriate size;
(ii) pressure generator, e.g. ball pump (hand pump) with a deflation valve;
(iii) Stopwatch.
(2) Procedure,-
Air leakage shall not exceed a pressure drop of 0.8 kPa/min (6 mmHg/min). Check compliance by means of the following test. If, because of technical reasons, the test as described in this sub clause cannot be performed, use an alternative test procedure specified by the manufacturer.
Testing shall be carried out at environmental conditions.
Before beginning the test, allow the automated sphygmomanometer to reach working temperature.
Wrap the cuff around the cylinder such that, for devices measuring at the upper arm and the thigh, the circumference of the applied cuff does not exceed that of the cylinder by more than 7 %.
Carry out the test over the whole measurement range at at least three equally spaced pressure steps (e.g. 6.7 kPa (50 mmHg), 20.0 kPa (150 mmHg), and 33.3 kPa (250 mmHg)). Because the thermodynamic equilibrium is influenced by decreasing or increasing the pressure when changing to the next pressure step, wait at least 60 s before reading the values. Test the air leakage over a period of 5 minutes and determine the measured value from this.
Note 1: Electro-mechanical pumps which are a part of the system may be used for the test. Valves which are permanently opened may be disconnected for the test.
Note 2: For this test no calibrated reference manometer is required because the cuff pressure display of the unit under test can be used when the error of the cuff pressure indication is considered. The advantage of this test is that the unit under test is in its original configuration. Additional connections can increase the leakage.
(3) Expression of results,-
Express the air leakage as the rate of pressure loss per minute.
6. Test for pressure reduction rate of devices using the auscultatory method.-
To comply with the requirement of clause (ii) of sub-paragraph (4) of paragraph 6 of Part1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) T-piece connectors;
(ii) calibrated reference manometer with a signal output port and a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) artificial or human limbs (see Notes under 5(2) of Part 2);
(iv) recording unit.
(2) Procedure,-
Measure the pressure reduction rate either on human subjects or artificial limbs.
Note 1: The recommendation is to use artificial limbs, but measurements performed with
human volunteers are acceptable.
Note 2: Two limb sizes should be used, being equal to the upper and lower limits of the
limb circumferences with which a particular cuff size is recommended for use.
Note 3: It is recommended that the characteristics of the artificial limbs reflect some
elastic characteristics of human limbs.
Because the cuff deflation rate may be influenced by the way in which a cuff is applied,
apply and remove the cuff for each of at least ten repeated measurements on at least two
different limb sizes. The deflation may be reset.
Connect the calibrated reference manometer to the cuff by means of a T-piece. Connect
the output part of the calibrated reference manometer to the recording unit.
(3) Expression of results,-
Determine the rate of pressure reduction (e.g. by graphical evaluation and drawing tangents) at the pressure values 8.0 kPa (60 mmHg), 16.0 kPa (120 mmHg) and 24.0 kPa (180 mmHg). Calculate the pressure reduction rate as the mean value calculated separately for the pressure values 8.0 kPa (60 mmHg), 16.0 kPa (120 mmHg) and 24.0 kPa (180 mmHg) and for the various limb circumferences.
If the pressure reduction rate is dependent on the pulse, record the pulse rate. In this case, express the result as cuff deflation rate per pulse.
7. Test for rapid exhaust.-
To comply with the requirement of clause (iii) of sub-paragraph (4) of paragraph 6 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) two rigid vessels with capacities of 100 ml ± 5 ml and 500 ml ± 25 ml, respectively;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) pressure generator;
(iv) T-piece connector;
(5) Stopwatch.
(2) Procedure,-
Carry out the test with the 500 ml vessel in place of the cuff. For automated sphygmomanometers having the capability of measuring in a neonatal/infant mode and for devices measuring at the wrist, carry out the test with the 100 ml vessel in place of the cuff.
Connect the calibrated reference manometer by means of a T-piece to the pneumatic system.
Inflate at least to the initial pressure given in clause (iii) of sub-paragraph (4) of paragraph 6 of Part 1, wait 60 s and activate the rapid exhaust valve.
Measure the time between the pressure values specified in clause (iii) of sub-paragraph (4) of paragraph 6 of Part 1,using the stopwatch.
(3) Expression of results,-
Express the results as the measured exhaust time.
8. Test for zero adjustment of a measuring system.-
To comply with the requirement of clause (iv) of sub-paragraph (4) of paragraph 6 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) rigid vessel with a capacity of 500 ml ± 25 ml;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) electro-mechanical pressure/suction pump;
(iv) pressure generator, e.g. ball pump (hand pump) with a deflation valve;
(v) T-piece connectors;
(vi) hoses.
(2) Procedure and evaluation,-
If, for technical reasons, the test as described in this sub clause cannot be performed,
use an alternative test procedure specified by the manufacturer.
To test the function of the zero adjustment, apply a pressure of +0.8 kPa (+6 mmHg) and
subsequently –0.8 kPa (–6 mmHg) to the pneumatic system and initiate a zero-setting of the device.
Ensure that all displayed pressure values have a systematic error of –0.8 kPa (–6 mmHg)
and +0.8 kPa (+6 mmHg), respectively.
Before beginning the test, allow the automated sphygmomanometer to reach working temperature.
Set up the automated sphygmomanometer to be tested as follows:
(a) replace the cuff with the 500 ml vessel;
(b) insert the calibrated reference manometer into the pneumatic system by means of a T-piece connector;
(c) insert the pressure/suction pump into the pneumatic system by means of a T-piece connector;
(d) insert the pressure generator into the pneumatic system by means of a T-piece connector. Note: If convenient, one adjustable pump may be used in place of the pressure/suction pump and pressure generator to generate the pressures.
Proceed in the following way:
(i) perform a regular adjustment to zero on the automated sphygmomanometer;
(ii) raise the pressure to 13.0 kPa (or 100 mmHg) and record the indication of the automated sphygmomanometer (e.g. 12.9 kPa or 99 mmHg);
(iii) apply a pressure of +0.8 kPa (+6.0 mmHg) while performing another adjustment to zero;
(iv) raise the pressure to 13.0 kPa (or 100 mmHg) and record the indication of the automated sphygmomanometer. It shall be 0.8 kPa (6 mmHg) below the value recorded at (ii)) (e.g. 12.1 kPa or 93 mmHg);
(v) apply a pressure of –8 kPa (–6.0 mmHg) while performing another adjustment to zero;
(vi) raise the pressure to 13.0 kPa (or 100 mmHg) and record the indication of the automated sphygmomanometer. It shall be 0.8 kPa (6 mmHg) above the value recorded at (ii)) (e.g. 13.7 kPa or 105 mmHg).
(3) Expression of results,-
Express the results as shown in clause (iv) and clause (vi) of sub-paragraph (2) of paragraph 8 of this Part.
9. Test for instrumental drift of the cuff pressure indication.-
To comply with the requirement of clause (iv) of sub-paragraph (4) of paragraph 6 of Part 1, the following test shall be performed.
(1) General,-
This test applies for devices performing zero adjustment only immediately after switching on.
(2) Apparatus,-
The apparatus consists of the following:
(i) rigid vessel with a capacity of 500 ml ± 25 ml;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) stopwatch;
(iv) T-piece connectors;
(v) patient simulator as described in sub-paragraph(1) of paragraph 13 of this Part .
(3) Procedure and evaluation,-
Replace the cuff with the 500 ml vessel. Insert the calibrated reference manometer and the patient simulator into the pneumatic circuit by means of T-piece connectors.
Before beginning the test, allow the automated sphygmomanometer to reach operating temperature as described in the instructions for use.
Perform one blood pressure measurement, then determine the time, t, until the automated sphygmomanometer has switched off automatically.
Switch on the automated sphygmomanometer and set it to the test mode. Apply a pressure of 6.7 kPa (50 mmHg) according the procedure specified in test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity and start the stopwatch. Determine the change of the cuff pressure indication during the time, t. Check that it does not exceed 0.1 kPa or 1 mmHg.
10. Test for maximum time for which the cuff is inflated.-
To comply with the requirement of clause (vi) of sub-paragraph (4) of paragraph 6 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) patient simulator or human subject;
(ii) Stopwatch.
(2) Procedure and evaluation,-
Apply the automated sphygmomanometer to a human or connect it to the patient simulator. Simultaneously start a blood pressure measurement and the stopwatch. Extend the blood pressure measurement as long as possible. Examples (for automated sphygmomanometer measuring during cuff deflation) of how this can be achieved are:
(i) by moving the limb, which causes the cuff deflation to halt or re-inflate;
(ii) by manually blocking the deflation valve.
Measure the time until the cuff pressure drops below the pressure value specified in clause (vi) of sub-paragraph (4) of paragraph 6 of Part 1.
11. Test for durability.-
To comply with the requirement of of sub-paragraph (6) of paragraph 6 of Part 1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) rigid metal vessel with a capacity of 500 ml ± 25 ml;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) pressure generator, e.g. ball pump (hand pump) with a deflation valve;
(iv) T-piece connectors and hoses.
(2) Procedure,-
Carry out the test for the maximum permissible errors of the cuff pressure indication but only at a temperature of 20 °C ± 5 °C and at ambient humidity prior to prolonged usage.
Perform 10,000 simulated measurement cycles with the complete automated sphygmomanometer at which at least the following cuff pressure values shall be reached:
(i) adult mode: 20.0 kPa (150 mmHg);
(ii) neonatal/infant mode: 10.0 kPa (75 mmHg).
Note 1: For devices which measure with the auscultatory and oscillometric method this test should be carried out for both modes.
Note 2: For devices which measure in both modes (adult and neonatal/infant) the test should be carried out in both modes.
(3) Expression of results,-
Express the results as the difference between the cuff pressure indication before and after 10,000 simulated blood pressure measurement cycles at the same test pressure and under the same environmental conditions.
12. Test for signal input and output ports.-
To comply with the requirement of of sub-paragraph (8) of paragraph 6 of Part1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) rigid vessel with a capacity of 500 ml ± 25 ml;
(ii) calibrated reference manometer with a maximum permissible error within ±0.1 kPa (±0.8 mmHg);
(iii) T-piece connectors;
(iv) pressure generator, e.g. ball pump (hand pump) with a deflation valve.
(2) Procedure,-
Replace the cuff with the 500 ml vessel, insert the calibrated reference manometer into the pneumatic system by means of a T-piece and proceed as follows:
(i) raise the pressure to 13.3 kPa (100 mmHg) and record the displayed value;
(ii) repeat (i) whilst short circuiting all contacts of the signal input/output ports belonging to the automated sphygmomanometer;
(iii) repeat (i) whilst applying the maximum voltage specified by the manufacturer to each contact belonging to the automated sphygmomanometer.
(3) Evaluation,-
Compare the indicated value under clause (i) of sub-paragraph(2) of paragraph 12 of this Part with the indicated values under clause(ii) and (iii) of sub-paragraph(2) of paragraph 12 of this Part.
13. Test for cuff pressure deflation following an aborted measurement.-
To comply with the requirement of clause (i) of sub-paragraph (9) of paragraph 6 of Part1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) patient simulator or human subject.
(2) Procedure and evaluation,-
Apply the automated sphygmomanometer to a human or connect it to the patient simulator. Start a blood pressure measurement. Abort the measurement during inflation. Start another measurement and abort it during the pressure reduction. If interval measurements are possible repeat the test in this mode.
Check by visual inspection whether the rapid exhaust is activated.
14. Test for resistance to vibration and shock.-
To comply with the requirement of sub-paragraph (10) of paragraph 6 of Part1, the following test shall be performed.
(1) Apparatus,-
The apparatus consists of the following:
(i) shaker.
(2) Procedure,-
Compliance is checked by the following tests:
(i) Shock test using the conditions of test type 1 or 2:
(a) Test type: Type 1:-
(I) peak acceleration: 150 m/s2 (15 g);
(II) duration: 11 ms;
(III) pulse shape: half sine;
(IV) number of shocks: 3 shocks per direction per axis (18 total).
(b) Test type: Type 2:
(I) peak acceleration: 300 m/s2 (30 g);
(II) duration: 6 ms;
(III) pulse shape: half sine;
(IV) number of shocks: 3 shocks per direction per axis (18 total).
(V)
(ii) Broad-band random vibration using the following conditions
(a) Acceleration amplitude:
(I) 10 Hz to 100 Hz: 1.0 (m/s2)2/Hz;
(II) 100 Hz to 200 Hz: –3 dB/octave;
(III) 200 Hz to 2 000 Hz: 0.5 (m/s2)2/Hz;
(b) Duration: 30 min per each perpendicular axis (3 total).
(iii) Evaluation
After this test the automated sphygmomanometer shall comply with the requirements of sub-paragraph (1) of paragraph 5 of Part 1, but only at a temperature of 20 °C ± 5 °C and at ambient humidity.
15. Test for durability of markings.-
To comply with the requirement of sub-paragraph (11) of paragraph 6 of Part 1, the following test shall be performed:
Check compliance by inspection and the following tests:
After all the other tests of this document have been performed:
(i) markings are rubbed by hand, without undue pressure, first for 15 s with a cloth soaked with distilled water, then for 15 s with a cloth soaked with methylated spirits and then for 15 s with a cloth soaked with isopropyl alcohol;
(ii) adhesive labels shall not have worked loose or become curled at the edges.
[File No. WM-9(24)/2024]
(Anupam Mishra)
Joint Secretary to the Government of India
Note:- The principal rules were published in the Gazette of India, Extraordinary, Part II, Section3, Sub-section (i), Vide notification number G.S.R.71(E), dated the 7th February, 2011 and was last amended , vide notification G.S.R 763(E), dated the 4th October, 2022.




